Disease Drivers of Aging: The 2016 Advances in Geroscience Summit
Reported by:
Jennifer Cable
Presented by:
Gerontological Society of America
The New York Academy of Sciences
Overview
The processes associated with aging may be risk factors for age-related diseases. Conversely, many age-related chronic diseases accelerate aging. However, because most research is focused on specific diseases, the broad mechanisms of disease as a driver of aging have not been well studied. Understanding how disease drives aging could not only help people live longer but also improve health and quality of life during old age.
On April 13–14, 2016, researchers studying aging and three disease areas—HIV/AIDS, oncology, and diabetes—met at the New York Academy of Sciences for the Disease Drivers in Aging: 2016 Advances in Geroscience Summit. The meeting expanded on the National Institutes of Health’s 2013 Advances in Geroscience Summit, examining how chronic diseases and their treatments affect aging. The meeting also aimed to stimulate collaboration between researchers studying aging and researchers studying disease. The summit was presented by the Gerontological Society of America, the American Federation for Aging Research, the Trans-NIH GeroScience Interest Group, and the New York Academy of Sciences.
The meeting opened with four keynote presentations from geroscience researchers, followed by a series of short talks and panel discussions featuring researchers focused on HIV/AIDS, diabetes, and cancer. This eBriefing reviews the panel discussions on each disease area and the overarching discussion on common themes and ideas for collaboration between researchers studying aging and disease.
Presentations available from:
Timothy Ahles, PhD (Memorial Sloan Kettering Cancer Center)
Kathryn Anastos, MD (Albert Einstein College of Medicine)
Steven Austad, PhD (University of Alabama at Birmingham)
James Becker, PhD (University of Pittsburgh)
Caroline Blaum, MD (New York University)
Judith Campisi, PhD (Buck Institute for Research on Aging)
Harvey J. Cohen, MD (Duke University)
Wendy Demark-Wahnefried, PhD (University of Alabama at Birmingham)
Jan van Deursen, PhD (Mayo Clinic)
Elissa Epel, MD (University of California, San Francisco)
Kristine Erlandson, MD (University of Colorado)
Brooke Grindlinger, PhD (The New York Academy of Sciences)
Jeffrey Halter, MD (University of Michigan)
Richard Hodes, MD (National Institute on Aging, NIH)
Peter Hunt, MD (University of California, San Francisco)
Beth Jamieson, PhD (University of California, Los Angeles)
C. Ronald Kahn, MD (Joslin Diabetes Center)
Balakuntalam Kasinath, MD (University of Texas Health Science Center)
Gretchen Neigh, PhD (Virginia Commonwealth University)
Rosario Scalia, MD, PhD (Temple University)
Marissa Schafer, PhD (Robert and Arlene Kogod Center on Aging)
Felipe Sierra, PhD (National Institute on Aging, NIH)
Mary Sehl, MD, PhD (University of California, Los Angeles)
Christopher Wiley, PhD (Buck Institute for Research on Aging)
Panel Discussions
Presented by

How to cite this eBriefing
The New York Academy of Sciences. Disease Drivers of Aging: The 2016 Advances in Geroscience Summit. Academy eBriefings. 2016. Available at: www.nyas.org/Geroscience2016-eB
Media
04:25 2. What we know from basic research
11:58 3. Progress made; What we want to know; Conclusion
08:20 2. Chronic stress and cell aging
13:04 3. Telomeres and disease risk
15:55 4. Disease drivers; Conclusion
10:20 2. Presentation by James Becker
19:52 3. Presentation by Gretchen Neig
10:16 2. Presentaton by Peter Hunt
20:23 3. Presentation by Kristine Erlandson
08:25 2. HIV and macrophages; Primate studies; Where to intervene
16:21 3. Maximizing control group resources; Biomarkers for stress
23:48 4. Regarding Alzheimer’s; Mitochondrial dysfunction; Aging and viral dormancy; Data set creation
28:56 5. Supressor mutations; Cognitive dysfunction; Conclusion
11:07 2. Presentation by Christopher Wiley
22:09 3. Presentaton by Mary Sehl
10:29 2. Presentation by Balakuntalam Kasinath
20:47 3. Presentation by Rosario Scalia
10:35 2. Presentation by Jeffrey Halter
09:03 2. Increased phosphorylation; Obesity, asthma, and diabetes
13:55 3. Amino acids and insulin secretion; More on accelerated aging
18:52 4. Regarding astroglial cells; The blood-brain barrier
26:40 5. Treatment with metformin; Diabetes and immunodeficiency; Rapamycin; Conclusion
04:20 2. General manuscript preparation; Discussion and introduction
11:44 3. The abstract; The title
14:38 4. Authorship; The cover letter
21:44 5. Referees; Peer review
27:44 6. Decisions; Common reasons for rejection; Revision and resubmission
34:35 7. Preparing a response; Appealing a negative decision
39:12 8. Parting thoughts; Conclusion
11:48 2. Presentation by Harvey Jay Cohen
22:09 3. Presentation by Judith Campisi
10:29 2. Presentation by Timothy Ahles
07:48 2. Synergy between aging and cancer research models; Aging in animal models
17:30 3. Disease interplay across disciplines; Dietary restriction; Senescent T-cells
24:12 4. Computational and systems biology; Chemotherapy and senescence; Funding
31:11 5. Anti-depressants before chemo; Intervention
40:15 6. Regarding exosomes and evolution; Conclusion
13:17 2. Disease treatment and lifespan extension; Targeting senescenct cells
21:06 3. Embracing complexity; Developmental manipulation in aging research; Inflammation
33:20 4. Review and funding
41:00 5. Metabolic reprogramming; Healthspan vs. lifespan; Regarding data sets
48:18 6. Mitochondrial dysfunction; Going forward; Common tools
61:28 7. Aging as disease; Stress and trauma; Drug discovery; Influence of local environment
69:28 8. Exercise; Incentivizing collaboration; Policy and funding
82:06 9. Focus on gene expression; Big data; Public perceptions
87:27 10. Cohort development; Conclusion
Resources
General aging resources: websites
Frontiers in Geroscience. J Gerontol. 2014;69 Suppl 1
Supplemental issue of the Journals of Gerontology containing articles related to the first Geroscience Summit.
NIH Geroscience Interest Group
Trans-NIH organization to raise awareness of the role of aging in disease.
Sierra F, Kohanski R (eds). Advances in Geroscience. Springer International Publishing. 2016. [Ebook]
Provides a comprehensive overview of geroscience.
General aging resources: journal articles
National Institute on Aging (NIA)
NIA, one of the 27 Institutes and Centers of NIH, leads a broad scientific effort to understand the nature of aging and to extend the healthy, active years of life.
Bansal A, Zhu LF, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci U S A. 2015;112:E277-E286.
Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709-713.
Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1192-1217.
Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at end of life. JAMA. 2003;289:2387-2392.
St Sauver JL, Boyd CM, Grossardt BR, et al. Risk of developing multimorbidity across all ages in an historical cohort study: differences by sex and ethnicity. BMJ Open. 2015;5:e006413.
Research and funding opportunities: websites
Impact of Aging on Currently Employed Animal Models of Disease and Chronic Conditions
Recent NIA request for applications.
NIA Interventions Testing Program
Multi-site study that investigates the potential for treatments to extend life span and delay dysfunction in mice.
HIV and aging resources: journal articles
Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18 Suppl 1:S11-S8.
Cohen MH, Hotton AL, Hershow RC, et al. Gender-related risk factors improve mortality predictive ability of VACS Index among HIV-infected women. J Acquir Immune Defic Syndr. 2015;70:538-544.
Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633-645.
Lee SA, Sinclair E, Hatano H, et al. Impact of HIV on CD8+ T cell CD57 expression is distinct from that of CMV and aging. PLoS One. 2014;9:e89444.
Rickabaugh TM, Baxter RM, Sehl M, et al. Acceleration of age-associated methylation patterns in HIV-1-infected adults. PLoS One. 2015;10:e0119201.
Schrack JA, Althoff KN, Jacobson LP, et al. Accelerated longitudinal gait speed decline in HIV-infected older men. J Acquir Immune Defic Syndr. 2015;70:370-376.
Diabetes and aging resources: journal articles
Chang AM, Smith MJ, Galecki AT, et al. Impaired beta-cell function in human aging: response to nicotinic acid-induced insulin resistance. J Clin Endocrinol Metab. 2006;91:3303-3309.
Cheng Z, Jiang X, Pansuria M, et al. Hyperhomocysteinemia and hyperglycemia induce and potentiate endothelial dysfunction via µ-calpain activation. Diabetes. 2015;64:947-959.
Han BH, Blaum CS, Ferris RE, et al. Older adults reporting more diabetes mellitus care have greater 9-year survival. J Am Geriatr Soc. 2015;63:2455-2462.
Kleinridders A, Cai W, Cappellucci L, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci U S A. 2015;112:3463-3468.
Mariappan MM, Prasad S, D’Silva K, et al. Activation of glycogen synthase kinase 3β ameliorates diabetes-induced kidney injury. J Biol Chem. 2014;289:53563-35375.
Cancer and aging resources: journal articles
Bouchlaka MN, Sckisel GD, Chen M, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med. 2013;210:2223-2237.
Dale W, Mohile SG, Eldadah BA, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst. 2012;104:581-589.
Lecot P, Alimirah R, Desprez PY, et al. Context-dependent effects of cellular senescence in cancer development. Br J Cancer. 2016. [Epub]
Mandelblatt JS, Jacobsen PB, Ahles T. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol. 2014;32:2617-2626.
Mohile SG, Hurria A, Cohen HJ, et al. Improving the quality of survivorship for older adults with cancer. Cancer. 2016.
Van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439-446.
Speakers
Organizers
James Appleby, RPh, MPH
The Gerontological Society of America
website
Steven Austad, PhD
University of Alabama at Birmingham
website | publications
Rita Effros, PhD
University of California, Los Angeles
website | publications
Rebecca Fuldner, PhD
National Institute on Aging, NIH
website | publications
Paige Green, PhD, MPH
National Cancer Institute, NIH
website | publications
Robin Huebner, PhD, MPH
National Institute of Allergy and Infectious Diseases, NIH
Ronald Kohanski, PhD
National Institute on Aging, NIH
website | publications
Stephanie Lederman, EdM
American Federation for Aging Research
website | publications
Kevin J. Lee, PhD
The Lawrence Ellison Foundation; American Federation for Aging Research
website
Judie Lieu
The Gerontological Society of America
website
Francesca Macchiarini, PhD
National Institute of Allergy and Infectious Diseases, NIH
website | publications
Susan McCarthy, PhD
National Cancer Institute, NIH
website | publications
Aaron Pawlyk, PhD
National Institute of Diabetes and Digestive and Kidney Diseases, NIH
website | publications
Felipe Sierra, PhD
National Institute on Aging, NIH
website | publications
Luke Stoeckel, PhD
National Institute of Diabetes and Digestive and Kidney Diseases, NIH
website | publications
Odette van der Willik
American Federation for Aging Research
website | publications
Siobhan Addie, PhD
Formerly at the New York Academy of Sciences
Brooke Grindlinger, PhD
The New York Academy of Sciences
Speakers
Timothy Ahles, MD
Memorial Sloan Kettering Cancer Center
website | publications
Kathryn Anastos, MD
Albert Einstein College of Medicine
website | publications
Nir Barzilai, MD
Albert Einstein College of Medicine
website | publications
James Becker, PhD
University of Pittsburgh
website | publications
Caroline Blaum, MD
New York University
website | publications
Judith Campisi, PhD
Buck Institute for Research on Aging
website | publications
Harvey J. Cohen, MD
Duke University
website | publications
Steven Deeks, MD
AIDS Research Institute; University of California, San Francisco
website | publications
Wendy Demark-Wahnefried, PhD, RD
University of Alabama at Birmingham
website | publications
Jan van Deursen, PhD
Mayo Clinic
website | publications
Elissa Epel, MD
University of California, San Francisco
website | publications
Kristine Erlandson, MD
University of Colorado
website | publications
Claudia Gravekamp, PhD
Albert Einstein College of Medicine
website | publications
Jeffrey Halter, MD
University of Michigan
website | publications
Kevin High, MD
Wake Forest University
website | publications
Richard Hodes, MD
National Institute on Aging, National Institutes of Health
website | publications
Peter Hunt, MD
University of California, San Francisco
website | publications
Arti Hurria, MD
City of Hope National Medical Center
website | publications
Beth Jamieson, PhD
University of California, Los Angeles
website | publications
C. Ronald Kahn, PhD
Joslin Diabetes Center
website | publications
Balakuntalam Kasinath, MD
University of Texas Health Science Center
website | publications
Gretchen Neigh, PhD
Virginia Commonwealth University
website | publications
Marissa Schafer, PhD
Mayo Clinic College of Medicine
publications
Rosario Scalia, MD, PhD
Temple University
website | publications
Reported by:
Jennifer Cable
Jennifer Cable lives in New York City, where she experiments with different outlets to communicate science. She enjoys bringing science to scientists and nonscientists alike. She writes for Nature Structural and Molecular Biology, Bitesize Bio, Under the Microscope, and the Nature New York blog. She received a PhD from the University of North Carolina at Chapel Hill for her research in investigating the structure/function relationship of proteins.
Sponsors
Silver Sponsor
Academy Friends
American Federation for Aging Research
The Gerontological Society of America
Grant Support
Funding for the eBriefing of this conference was made possible, in part, by 1 R13 AG 053043-01 from the National Institute on Aging. Co-funding has been provided by the National Institute of Diabetes and Digestive and Kidney Diseases, and the National Cancer Institute. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
This activity is supported by an educational grant from Lilly. For further information concerning Lilly grant funding visit www.lillygrantoffice.com
Regeneron Pharmaceuticals, Inc.
Presented by

Keynote Presentations
Keynote Speakers
Richard Hodes
National Institute on Aging, NIH
Felipe Sierra
National Institute on Aging, NIH
Steven Austad
University of Alabama at Birmingham
Elissa Epel
University of California, San Francisco
Highlights
- Although age is an important variable in clinical research, the effects of aging are often understudied.
- Improving the quality of life during aging is as important as increasing longevity.
- Short-term stress can promote resilience to aging; however, chronic stress accelerates aging.
Introduction
Aging is often accompanied by chronic diseases including heart disease, cancer, diabetes, and Alzheimer’s disease, to name a few. Many processes associated with aging may be risk factors for these diseases. While aging occurs in the absence of disease—a steady decline in health is expected as a person ages—chronic diseases also accelerate aging, exacerbating normal physical and cognitive decline.
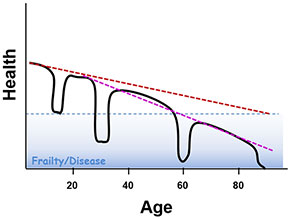
The field of geroscience has focused on how aging affects disease, and this topic was the theme of the first geroscience summit, “Advances in Geroscience: Impact on Healthspan and Chronic Disease,” which took place in October, 2013, in Bethesda, Maryland. Papers related to that summit were published in a supplement to the Journals of Gerontology in June, 2014. However, the reverse question—how disease affects aging—has gone largely unstudied, and this topic was the theme of this 2016 meeting.
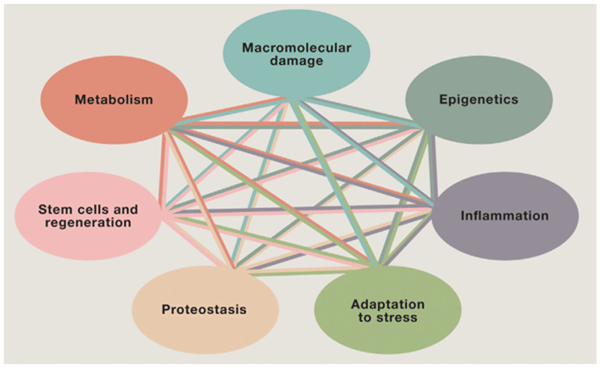
The 2016 summit examined how three diseases—HIV/AIDS, diabetes, and cancer—affect the major pillars of aging established at the first Geroscience Summit: macromolecular damage, stress response, epigenetics, stem cells, proteostasis, inflammation, and metabolism.
The meeting opened with four keynote presentations from geroscience researchers who described successful interventions to delay aging and increase life span in animal models. They also noted the need for multidisciplinary research, challenging attendees to work across disease states. Understanding common mechanisms in aging and chronic diseases could help people live not only longer but also healthier lives.The keynote presentations were followed by a series of short talks and panel discussions featuring researchers focused on HIV/AIDS, diabetes, and cancer. This eBriefing reviews the panel discussions on each disease area and the overarching discussion on common themes and ideas for collaboration between researchers studying aging and disease.
A multidisciplinary approach to aging
Richard Hodes of the National Institute of Aging (NIA) outlined the multidisciplinary structure at the NIA and National Institutes of Health (NIH) to raise awareness of the role of aging in disease and to fund aging-based research. Targeting aging may reduce the incidence of age-related diseases. The divisions of the NIA span basic biology to clinical and behavioral studies. Because aging touches so many fields, the NIH also created the Geroscience Interest Group (GSIG), with members from 21 institutes. Hodes called on researchers to step outside the silos of their specialties and to study features of aging common to disparate disciplines and diseases.
Hodes also presented evidence of the importance of considering age when choosing treatments for patients, and highlighted the need to include elderly subjects in preclinical and clinical studies. In the Diabetes Prevention Program, both medication and lifestyle changes reduced the risk of developing diabetes in at-risk patients younger than 60 years. However, in subjects older than 60, only lifestyle changes were effective. In a pre-clinical study, elderly mice with cancer died sooner when given immunotherapy than those who did not receive treatment.
Felipe Sierra, also from the NIA, pointed to the need to re-examine our approach to disease. The elderly often present with several comorbidities. Instead of considering each disease individually, researchers should consider interactions between diseases, and between disease and aging.
The keynote speakers also described efforts to promote aging research. In late 2015, the NIA released a request for applications to test whether the age of laboratory animals affects outcomes in studies investigating disease. In the NIA’s Interventions Testing Program (ITP), researchers can submit compounds to be tested for an effect on extending life span in mice.
Increasing health span as well as life span
Steven Austad of the University of Alabama at Birmingham emphasized that increasing longevity is not the same as improving health. Several interventions can extend life span in animals. In some cases, such as the use of rapamycin in mice, health span is also improved. Indeed, rapamycin extends life and delays Alzheimer’s disease, cancer, atherosclerosis, and heart dysfunction in mice. However, genetic mutations that increase life span in the worm Caenorhabditis elegans do not extend the period of good health. Furthermore, life-extending interventions are not without side effects. In mice, dietary restriction and rapamycin have been linked to low bone density, immunosuppression, cataracts, diabetes, and hyperlipidemia.
Longer human life expectancy with improved treatments for fatal diseases often comes at the cost of declining quality of life and increased disability—Alzheimer’s disease, dementia, frailty, vision loss, pain, joint replacement, and other conditions. Treating the underlying causes of aging may prevent this deterioration in quality of life.
Telomere length as a marker of aging
Elissa Epel of the University of California, San Francisco, discussed the relationship between stress and cell aging. Epel uses telomere length—stretches of repeat DNA at the end of chromosomes—as an indicator of aging. Chronic disease can accelerate telomere attrition; while there are myriad biochemical alterations in each disease, many diseases of aging feature the triad of oxidative stress, inflammation, and hyperglycemia / insulin resistance. For example, in diabetes the disease processes of impaired β-cell function, and resulting higher levels of biochemical stressors, further shorten telomeres.
Psychiatric diseases, particularly major depression and anxiety disorders, are also associated with shortened telomeres. Given the high comorbidity of medical and psychiatric conditions, Epel noted, it is important to consider that depression may affect aging biology, not just the physical disease condition. Treatments for diseases may further affect the rate of telomere attrition, either speeding it up or slowing it down. For example, statins and possibly metformin, used in diabetes treatment, may prevent telomere attrition, while HAART therapy in HIV may accelerate telomere attrition. Chemotherapy can work through telomere damage of cancerous as well as healthy cells. Childhood traumas followed by chronic stressors in adulthood result in shortened telomeres, which correlate with several diseases of aging, including diabetes, cardiovascular disease, stroke, and cancer. Shortened telomeres might be both a cause and a consequence of chronic disease, although this has yet to be fully confirmed.
Disease Drivers of Aging: HIV, Diabetes, and Cancer
Chairs
Ronald Kohanski
National Institutes on Aging and Geroscience Interest Group, NIH
Steven Deeks
AIDS Research Institute; University of California, San Francisco
Claudia Gravekamp
Albert Einstein College of Medicine
Arti Hurria
City of Hope National Medical Center
Jeffrey Halter
University of Michigan
Kevin High
Wake Forest University
Nir Barzilai
Albert Einstein College of Medicine
Highlights
- Each person has a unique fingerprint of chronic viral infections—HIV is one example.
- HIV treatment normalizes some markers of aging.
- Some manifestations of diabetes are relevant to aging, others are not.
- Insulin resistance in the brain may accelerate age-related cognitive impairment.
- Both cancer and cancer therapies promote cellular senescence and inflammation, which contribute to aging and disease.
- Cancer treatment affects areas of the brain that are also changed by aging; these treatments can promote cognitive decline.
Aging with HIV
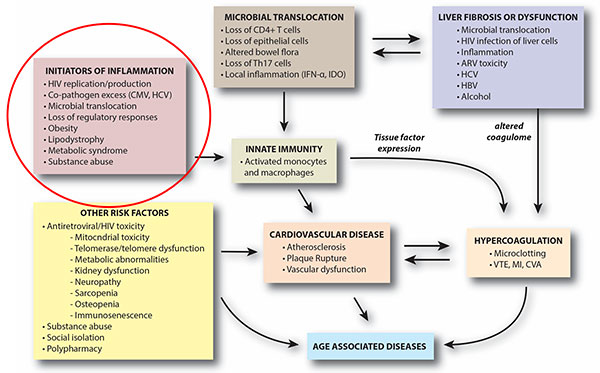
Despite the success of antiretroviral (ARV) therapy in improving health and increasing life span, people living with HIV are at an increased risk for many age-related diseases, such as cardiovascular disease, cancer, osteoporosis, liver disease, and cognitive impairment. Several panelists stressed the need for a multidimensional approach to investigate HIV and aging, saying that researchers should consider interactions between the seven pillars of aging described during the keynote presentations and focus on possible root causes driving aging.
Although the molecular pathways of aging in people living with HIV are well understood, the field lacks high-quality biomarkers to assess the effectiveness of interventions in clinical trials. The panelists advised aging researchers to make use of the large cohorts established in HIV, particularly MACS, WIHS, and VACS. Researchers have been following both infected and uninfected subjects in these cohorts for up to 30 years, and several types of samples, including serum, DNA, plasma, and urine, are available for study.
Gretchen Neigh of Virginia Commonwealth University discussed social stress and HIV. Her work in a rhesus monkey model, used to mimic human infection, showed that previous social stress increased the viral load of simian immunodeficiency virus (SIV) and delayed ARV response. The audience was curious as to whether this effect was attributable to the virus itself or to the illness that SIV causes. This question is difficult to answer in current animal models. Neigh added that the rhesus monkey model could be useful for studying the psychosocial aspects of HIV infection and for studying how social stressors affect viral reservoirs.
Beth Jamieson of the University of California, Los Angeles, showed that ARV therapy can partially normalize DNA methylation patterns, a hallmark of aging. She would like to explore this area further to find out how factors such as duration of infection and early treatment influence this effect. One attendee suggested targeting mitochondrial dysfunction to mitigate the effects of HIV on aging. The panelists pointed to the dearth of studies linking mitochondrial dysfunction to end-organ disease and noted that several older ARVs are mitochondrial toxins, complicating such analyses. Another area of investigation is infection of macrophages by HIV. While most of the panelists focused on infection of T cells, HIV infects macrophages during later stages of disease and could be a source of persistent inflammation.
The effects of type 2 diabetes on aging
It is difficult to establish which complications of type 2 diabetes accelerate age-related effects and which are specific to hyperglycemia, unrelated to aging. For example, patients with diabetes may experience vascular complications, such as neuropathy and nephropathy. However, these effects do not occur with normal aging and are likely a specific consequence of hyperglycemia. However, as Nir Barzilai of the Albert Einstein College of Medicine reminded attendees, researchers are not starting from scratch. Metformin, a common diabetes medication, also prevents several age-related diseases. Understanding this mechanism could be a starting point from which to investigate the effect of diabetes on age-related diseases.
Several audience members asked about the role of insulin resistance in the brain. During his presentation, C. Ronald Kahn of the Joslin Diabetes Center showed that knocking out the insulin receptor in the brain increased measures of anxiety in elderly mice but not in young mice. Kahn’s data also showed the importance of astroglia in insulin signaling. Attendees asked whether there were different families or subsets of astroglia in terms of insulin receptor expression or whether the cells had increased epigenetic variance with age. Kahn replied that he has not seen either effect, but added that he had not specifically looked for them.
One attendee questioned whether a homozygous knockout, which eliminates insulin signaling, is the best model for diabetes, noting that patients with diabetes retain some insulin signaling. Kahn replied that a heterozygous model may better recapitulate the biology of insulin resistance and that it may be worthwhile to use such a model.
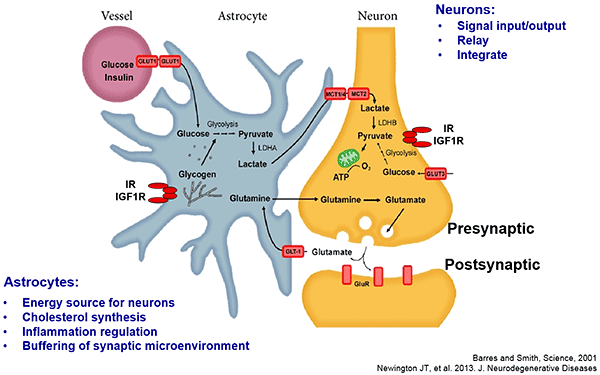
Another attendee asked about the use of CDK4 inhibitors, which are approved to treat some types of cancer. Jeffrey Halter of the University of Michigan showed that knocking out CDK4 in mice destroys β cells, which are responsible for producing insulin. Halter suggested that oncologists monitor for signs of β-cell dysfunction in patients taking CDK4 inhibitors for cancer. In addition, data from clinical trials of CDK4 inhibitors could be assessed retrospectively to evaluate whether these drugs have negative effects on β-cell function in humans.
One attendee asked the panel about the effects of asthma and decreased oxygen in obese people with diabetes and asthma. While no one on the panel has specifically investigated this relationship, the panelists cited studies in obese diabetics in which sleep apnea was shown to exacerbate insulin resistance. Improving sleep apnea decreased insulin resistance and diabetes.
Diabetes causes a subtle immunodeficiency. In other immunodeficiency models, such as HIV and transplant, cytomegalovirus (CMV) has been shown to play a role in driving vascular disease. One attendee postulated that CMV infection could be a good model to study the interaction between diabetes and aging in terms of vasculature, leukocytes, and endothelial function.
Premature aging caused by cancer and chemotherapy
Jan van Deursen of the Mayo Clinic explained that, in addition to apoptosis, chemotherapy can also induce cellular senescence in tumor cells. The panelists further explained that senescent tumor cells can be cleared by the immune system and that their ability to persist depends on the type of chemotherapy administered.
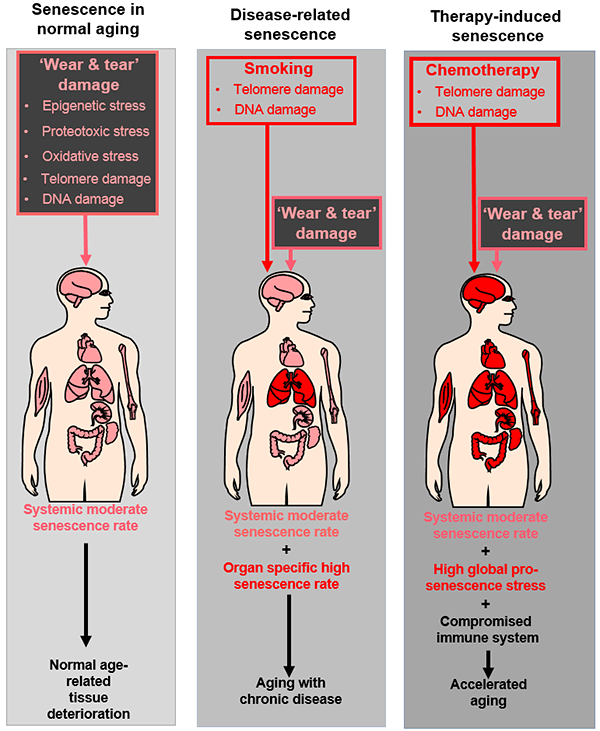
Timothy Ahles of Memorial Sloan Kettering Cancer Center described how cancer and its treatment result in cognitive decline. One attendee remarked on how brain imaging in normal aging has evolved to not only monitor damage but also to look at compensatory mechanisms that may result from more serious brain damage. Ahles explained that cancer researchers have learned a lot from imaging studies of both normal aging and cognitive impairment because many of the systems affected by normal aging are also affected by cancer treatment. Wendy Demark-Wahnefried of the University of Alabama at Birmingham presented data on accelerated adverse body composition changes that occur as a result of cancer treatment, with chemotherapy inducing gains in fat and losses in muscle in the year following diagnosis that are comparable to 10 years of normal aging.
There are many good mouse models of cancer, but the disease is often studied in young animals, and studies using these models cannot investigate how aging affects cancer. The panelists noted that there has been a push in the research community to use models that develop cancers more slowly, and for researchers using models to induce cancer in older animals. However, achieving these goals can be difficult because it is time consuming and costly to maintain elderly animals. To defray some of these costs, the trans-NIH Geroscience Interest Group (GSIG) put out an RFA, joined by several other Institutes including NCI, to age animal models of disease. Funding for these research projects will be provided jointly by NIA and other relevant NIH institutes. The panelists encouraged researchers to look outside mouse models; a recent review described studies of naturally occurring cancers in pet dogs. The panelists also suggested studying cancer in nonhuman primates.
Several attendees asked about how to mitigate the effects of chemotherapy. Some patients undergo short-term fasting before chemotherapy, which has been shown to promote tumor shrinkage in mice. However, researchers have not studied whether this strategy promotes longevity, similar to the effect of dietary restriction in mice. Similarly, researchers have not systematically studied the effects of antidepressant use before chemotherapy, which has been shown to prevent negative cognitive outcomes of chemotherapy in animal models.
The panelists recommended early interventions, such as exercise during chemotherapy, to prevent or reverse decline in physical function in cancer patients. However, they also noted that later interventions can be effective. In the RENEW trial, an international sample of 641 older, long-term cancer survivors at least 5 years out from diagnosis who received materials and counseling on good dietary and exercise habits had a slower decrease in physical function than those who did not. In another initiative, cancer survivors paired with a gardener had healthier habits, improved function, and improved biomarkers of aging after one year of gardening.
The Way Forward
Moderator
Felipe Sierra
National Institute on Aging, NIH
Highlights
- There is a need for a uniform definition of accelerated aging and standardized biomarkers to monitor aging.
- Researchers should identify and focus on the root causes that drive aging instead of downstream effects.
- Funding agencies should ensure that suitable aging-related experiments are included in grant proposals.
A call for uniformity
The meeting ended with a panel discussion on common themes and open research questions to advance therapies for chronic diseases and aging. Several attendees mentioned the need for a uniform definition of accelerated aging. There are many ways to define aging, ranging from molecular and cellular processes to functional ability and population age. In many animal models of aging, life span is the primary outcome for aging. However, this outcome would not be informative for clinicians; it does not reveal whether a patient is experiencing accelerated or normal aging or whether a treatment is improving the effects of aging. One attendee suggested creating an NIH toolbox of biological markers of aging to provide a common vocabulary for researchers. Currently, the best biomarkers of aging are composite measures that usually include function. The panelists recommended that studies measuring aging should include data representing at least two pillars of aging, such as DNA methylation and resistance to a stressor.
Several attendees also suggested that the research community create a single database, such as those housed at WikiPathways, to organize the biological pathways involved in aging and disease. Websites that currently house this information for aging do not include information on the relationships between aging and disease.
The big picture
Several panelists noted the importance of interactions between the seven pillars of aging. However, they cautioned against focusing on any single pillar and advised that the pillars be used as a guide and not as rigid categories for aging research. It is nonetheless clear that cellular senescence and inflammation are among the most important features of aging common to the three diseases discussed at this meeting. Cellular senescence has many forms, and targeting one form may not prevent others. Several attendees pointed out that therapeutic targeting of any one marker of inflammation or cellular senescence may not remedy their underlying cause(s) and their subsequent effects on aging and disease.
Funding and resources
The panelists also noted the need for more aging studies in general. One reason that investigators are hesitant to study aging is the time and expense of using aged animal models. While some larger centers, such as the Pepper Institute at Florida State University and the Shock Center at the University of Washington, have the infrastructure to do so, smaller institutions do not. Audience members recommended that the facilities at these centers be made available to researchers, particularly junior faculty, to help recruit more people to the aging field. The panelists also noted that datasets of relevance to aging are available on the NIA website.
Another reason for the lack of aging studies is the difficulty of acquiring funding for long-term, complex studies. The panelists remarked that grant reviewers sometimes ask that aging-related experiments be removed from proposals. Instead, aging experts on review committees should ensure that such complexity is included in proposals and confirm that an appropriate model system is being used so that studies on diseases that affect the elderly are not conducted in young mice. One audience member suggested supplements to existing grants be made available to fund extra samples for aging research. For example, ancillary studies could make use of specimens collected in clinical trials to analyze predictors of aging.
Open Questions
General
How can we best bring together those who study disease and those who study aging?
How should clinicians define aging and accelerated aging?
What biomarkers can be used to evaluate accelerated aging in the presence of chronic diseases and to assess the effects of interventions?
What are the best animal models to study accelerated aging in the presence of chronic disease?
Are life-extending therapies that work in animal models relevant to humans, and will they also extend health?
How can funding agencies, such as the NIA, encourage investigators to consider the effects of aging in their model systems?
Effects of HIV on aging
What is the role of senescent T cells in age-related diseases?
What can epigenetic changes in T cells tell us about the link between these cells and diseases of aging?
How does psychosocial stress affect HIV pathogenesis and response to treatment in humans?
What information can be gleaned from established HIV cohorts with respect to HIV and aging?
Future research questions for HIV and aging
How do disease factors, such as duration of infection and time of treatment, influence the effects of ARV therapy on markers of aging?
What can primate models of HIV infection tell us about psychosocial aspects of disease and how social stressors affect disease reservoirs?
What role might mitochondrial dysfunction play in HIV’s effect on aging?
How does infection of macrophages contribute to inflammation?
Effects of diabetes on aging
How do some diabetes therapies (eg, metformin and acarbose) increase longevity?
Which complications of diabetes are relevant to aging, and which are specific to increased blood glucose?
Could insulin resistance be a protective mechanism against accelerated aging?
Future research questions for diabetes and aging
How do CDK4 inhibitors affect β-cell function?
How do conditions that decrease the supply of oxygen, such as asthma and sleep apnea, influence insulin resistance?
What role might the immunodeficiency caused by diabetes play in driving vascular dysfunction?
Effects of cancer on aging
What are the different signs of, effects of, and treatments for cancer-related and chemotherapy-related senescence?
How can we definitively show that inflammation and coagulation affect function and survival?
Future research questions for cancer and aging
Can lifestyle changes improve cognitive function in older cancer patients?
How might short-term fasting prior to chemotherapy affect aging?
Can taking antidepressants before initiating chemotherapy mitigate cognitive decline?



