Cancer Immunotherapy: Advances in Combination Therapies
Reported by:
Jordana Thibado
Presented by:
Cancer Discussion Group
The New York Academy of Sciences
Overview
Immunotherapy, which harnesses the immune system to target and attack cancer cells, has emerged as a revolutionary approach to cancer treatment. Despite its effectiveness for a wide range of cancers, eligibility for treatment is dependent on the patient’s immune system, age, and genetic profile, as well as how advanced the cancer is and whether it has responded to prior treatment. Current research aims to improve treatment strategies and better predict patient outcomes. On May 11-12, 2020, the New York Academy of Sciences hosted the annual Frontiers in Cancer Immunotherapy symposium. Experts in tumor immunology, cancer genetics, and computational biology discussed novel therapeutic targets, tumor evolution, and the mechanisms driving resistance to current treatment. Learn about the latest research advances in cancer immunotherapy in this summary.
Symposium Highlights
- Computational neoantigen model predicts cancer patient outcomes.
- Combination immunotherapy produces new T cells that extend patient survival.
- Personalized cancer vaccines demonstrate therapeutic benefits.
- New computational genomics tools provide pediatric cancer insight.
- Immune checkpoint inhibitor associated toxicity yields new clinical syndrome.
- Macrophage reprogramming can halt metastatic cancer progression.
- Immune cell “neighborhoods” are altered during cancer progression.
- Multi-specific CAR T cell therapy may reduce immunotherapy resistance.
Speakers
James Allison, PhD
MD Anderson Cancer Center
Mikala Egeblad, PhD
Cold Spring Harbor Laboratory
Benjamin Greenbaum, PhD
Memorial Sloan Kettering Cancer Center
Crystal Mackall, MD
Stanford University
Elaine Mardis, PhD
Nationwide Children’s Hospital
Garry Nolan, PhD
Stanford University
Jeffrey Sosman, PhD
Feinberg School of Medicine, Northwestern University
Catherine Wu, MD
Dana-Farber Cancer Institute and Harvard Medical School
Event Sponsor
Advances Immune Checkpoint Therapies
Speakers
James Allison, PhD
MD Anderson Cancer Center
Benjamin Greenbaum, PhD
Memorial Sloan Kettering Cancer Center
Modeling Immune-Mediated Evolution
Benjamin Greenbaum examines immune interactions that contribute to cancer progression. His lab uses statistical physics and computational biology to study the role of neoantigens in tumor evolution. Neoantigens are mutated peptides present on the surface of cancer cells that have the potential to be immunogenic, meaning T cell receptors can recognize them for an immune response. The Greenbaum lab developed a mathematical model to understand the role of neoantigens. “The goal of this framework is to try to quantify the immunogenicity of neoantigens in an evolutionary model to better predict response to therapy,” said Greenbaum.
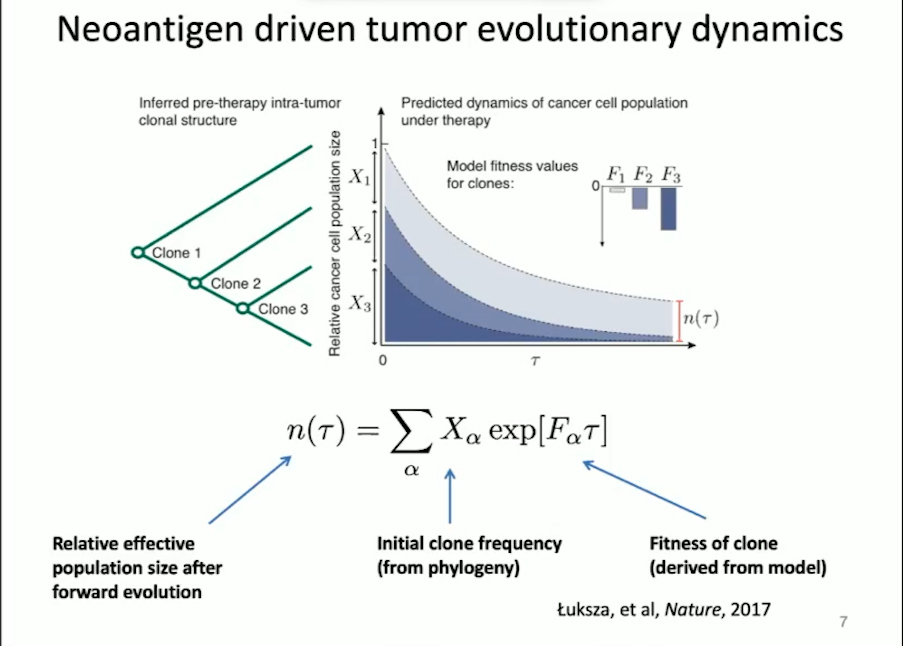
In this model, the tumor is characterized by the presence of neoantigens that are likely to provoke an immune response as well as the likeliness of that neoantigen progressing in forward evolution. Using this approach, the Greenbaum lab was able to separate patients who responded to treatment versus those who did not for both melanoma and small-cell lung cancer. The team will apply this model to predict patient outcomes in the clinic.
Immune Checkpoint Blockade in Cancer Therapy: Historical Perspective, New Opportunities
A pioneer in the field of cancer immunotherapy, James Allison has devoted his career to studying T cell response regulation and developing strategies for cancer treatment. In 2018, he was awarded the Nobel Prize in Physiology or Medicine jointly with Tasuku Honjo “for their discovery of cancer therapy inhibition of negative immune regulation,” as well as the Dr. Paul Janssen Award for Biomedical Research. One of Allison’s primary research areas is the role of immune checkpoint molecules, which help prevent the immune system from attacking healthy tissue. Early work in the Allison lab found that blocking a major immune checkpoint protein, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), allows T cells to target and destroy tumors. The FDA approved anti-CTLA-4 therapy, which demonstrated success in clinical trials. The effects have been significant: in metastatic melanoma, a disease with a median survival of seven months, anti-CTLA-4 therapy extended the survival of approximately 20% of patients by ten years after a single round of treatment.
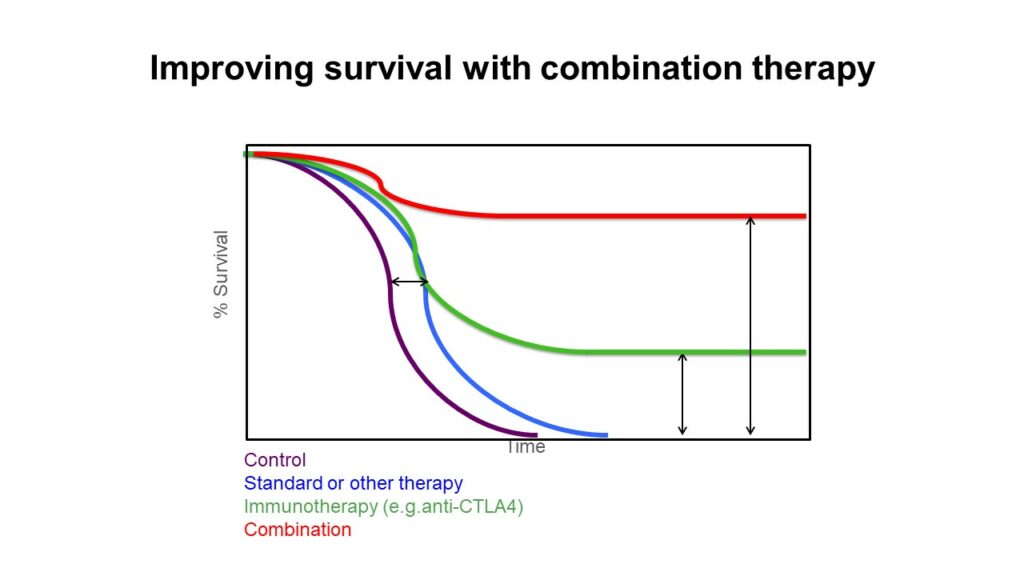
However, Allison wondered why this treatment wasn’t successful for all patients. After the discovery of another checkpoint inhibitor protein, programmed cell death protein 1 (PD1), Allison’s lab decided to use mass spectrometry to examine T cell populations before and after anti-CTLA-4 therapy, anti-PD1 therapy, and therapy combining both of these treatments. Although new T cell phenotypes arise following all three treatments, the combination therapy produces a synergistic effect that leads to the greatest increase in particular T cells that help target tumors. “It seems to be this that is responsible for the increase in efficacy, not just the combination of the effects of the monotherapies alone,” said Allison. His future work aims to enhance our understanding of combination checkpoint blockade therapy to extend the lives of more patients.
Further Readings
Greenbaum
Dudley JC, Lin M-T, Le DT, Eshleman JR
Microsatellite Instability as a Biomarker for PD-1 Blockade
Clin Cancer Res. 2016;22(4):813-820
Zhang J, Caruso FP, Sa JK, et al
Commun Biol. 2019;2:135
Balachandran VP, Łuksza M, Zhao JN, et al
Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer
Nature. 2017;551(7681):512-516
Łuksza M, Riaz N, Makarov V, et al
A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy
Nature. 2017;551(7681):517-520
Allison
Wei SC, Anang N-AAS, Sharma R, et al
Proc Natl Acad Sci USA. 2019;116(45):22699-22709
O’Shea JJ, Paul WE
Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells
Science. 2010;327(5969):1098-1102
Wei SC, Sharma R, Anang N-AAS, et al
Immunity. 2019;50(4):1084-1098.e10
Wei SC, Levine JH, Cogdill AP, et al
Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade
Cell. 2017;170(6):1120-1133.e17
Topalian SL, Hodi FS, Brahmer JR, et al
Safety, activity, and immune correlates of anti-PD-1 antibody in cancer
N Engl J Med. 2012;366(26):2443-2454
Schadendorf D, Hodi FS, Robert C, et al
J Clin Oncol. 2015;33(17):1889-1894
Leach DR, Krummel MF, Allison JP
Enhancement of antitumor immunity by CTLA-4 blockade
Science. 1996;271(5256):1734-1736
Beyond Immune Checkpoint Inhibitors
Speakers
Elaine Mardis, PhD
Nationwide Children’s Hospital
Catherine Wu, MD
Dana-Farber Cancer Institute and Harvard Medical School
Driving T cells into Tumors: A Role for Personal Cancer Vaccines
Catherine Wu works to enable more specific tumor targeting. She explained that one critical challenge is increasing the number of patients—right now, approximately 30%—with lasting responses to immunotherapies. Therapeutic vaccines may provide a solution. Wu said that these vaccines would, “stimulate antigen-specific immunity against determinants that are expressed in the cancer, and in doing so, increase the breadth and diversity of tumor-specific T cells.” Toward this goal, her lab has developed personalized vaccines for patients. The process begins with identifying specific mutations in a patient’s tumor using DNA and RNA sequencing. Next, human leukocyte antigen (HLA) typing is performed, and then personalized HLA-binding peptides are predicted. Excitingly, these vaccines, in combination with checkpoint blockade therapy, have demonstrated success in high-risk melanoma and glioblastoma patients. To improve HLA predictions, her lab has also developed an approach using mass spectrometry to examine antigen processing. To date, they have expanded from 16 to 95 HLA alleles that cover different racial groups such that they have 95% global coverage. This work represents an unprecedented advancement in distinguishing tumor-presenting epitopes.
Immunogenomics and the TME in Pediatric CNS Cancers
Elaine Mardis uses genomics to understand cancer progression and advance immunotherapeutic treatments. Specifically, her work focuses on understanding pediatric cancers. Unlike adult cancers, pediatric cancers have few druggable targets and are generally less well understood. By using and innovating computational approaches to characterize the tumor microenvironment and cancer progression, the Mardis lab provides unprecedented insight into pediatric cancers. For example, patient tumors are analyzed using exome sequencing and RNA sequencing with multiple analyses. Mardis has also developed a platform for neoantigen prediction called pVACtools, which aims to identify neoantigens from genomic fusion breakpoints. This work led to the discovery that recurrent pediatric central nervous system cancers contain immune cells in the tumor microenvironment that have been recruited from the periphery. This likely provides an immune-suppressive context based on checkpoint blockade, but neoantigen alterations may allow for the immune system to be engaged. However, new approaches may be required to extend immunotherapy to pediatric cancers because there are significant differences in the immune system based on age.
Further Readings
Wu
Sarkizova S, Klaeger S, Le PM, et al
Nat Biotechnol. 2020;38(2):199-209
Abelin JG, Keskin DB, Sarkizova S, et al
Immunity. 2017;46(2):315-326
Purroy N, Wu CJ
Coevolution of leukemia and host immune cells in chronic lymphocytic leukemia
Cold Spring Harb Perspect Med. 2017;7(4)
Keskin DB, Anandappa AJ, Sun J, et al
Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial
Nature. 2019;565(7738):234-239
Ott PA, Hu Z, Keskin DB, et al
An immunogenic personal neoantigen vaccine for patients with melanoma
Nature. 2017;547(7662):217-221
Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ
Getting personal with neoantigen-based therapeutic cancer vaccines
Cancer Immunol Res. 2013;1(1):11-15
Mardis
Hundal J, Kiwala S, McMichael J, et al
pVACtools: A Computational Toolkit to Identify and Visualize Cancer Neoantigens
Cancer Immunol Res. 2020;8(3):409-420
Hundal J, Carreno BM, Petti AA, et al
pVAC-Seq: A genome-guided in silico approach to identifying tumor neoantigens
Genome Med. 2016;8(1):11
Newman AM, Liu CL, Green MR, et al
Robust enumeration of cell subsets from tissue expression profiles
Nat Methods. 2015;12(5):453-457
Berger MF, Mardis ER
The emerging clinical relevance of genomics in cancer medicine
Nat Rev Clin Oncol. 2018;15(6):353-365
Toxicity, Tumor Response, and Microenvironment
Speakers
Mikala Egeblad, PhD
Cold Spring Harbor Laboratory
Jeffrey Sosman, PhD
Feinberg School of Medicine, Northwestern University
Immune Related Toxicities: Mechanisms and Disease Outcome
Jeffrey A. Sosman is an oncologist and cancer immunotherapy researcher studying immunotherapy toxicity. Although cancer immunotherapies have transformed the therapeutic landscape, these treatments have a variety of toxic effects that limit treatment potential. Fairly common toxicities include rashes, joint and tissue diseases, colitis and hepatitis, while more rare toxicities include encephalitis and myocarditis. Dr. Sosman presented a case study on a patient that developed immune checkpoint inhibitor (ICI) associated myocarditis, which he defined as a new clinical syndrome. Immune checkpoint inhibitors prevent natural “brakes” in the immune system so that T cells can attack cancer cells. Although myocarditis is rare in ICI-treated patients (less than 0.1%), this toxicity has a 50% mortality rate. Sosman shared that onset of ICI-associated myocarditis is highly unpredictable, though it can occur early on in treatment. He also noted that patients treated with combinations of immune checkpoint therapies are at higher risk for developing this toxicity. Future work aims to better predict serious toxicity and to understand the relationship between toxicity and tumor response.
Regulation of Cancer Progression by the Tumor Microenvironment
Mikala Egeblad studies the tumor microenvironment in cancer. Her lab has examined the role of bacterially derived lipopolysaccharides (LPS) in cancer progression, which is understood to influence macrophages within the tumor phenotypically. Specifically, the effect of LPS depends on the presence of either pro-inflammatory signals (IFNγ), which create tumoricidal macrophages, or anti-inflammatory signals (IL-4 and -13, TGFβ), which produce tumor-promoting macrophages. However, LPS cannot be used to treat cancer because of its toxicity in humans. Considering a novel therapeutic approach, Egeblad said, “we wanted to see if we could take tumor-promoting macrophages and reprogram them to become tumoricidal.” Their approach involved the use of monophosphoryl lipid A (MPLA), an LPS derivative that is already used as a vaccine adjuvant.
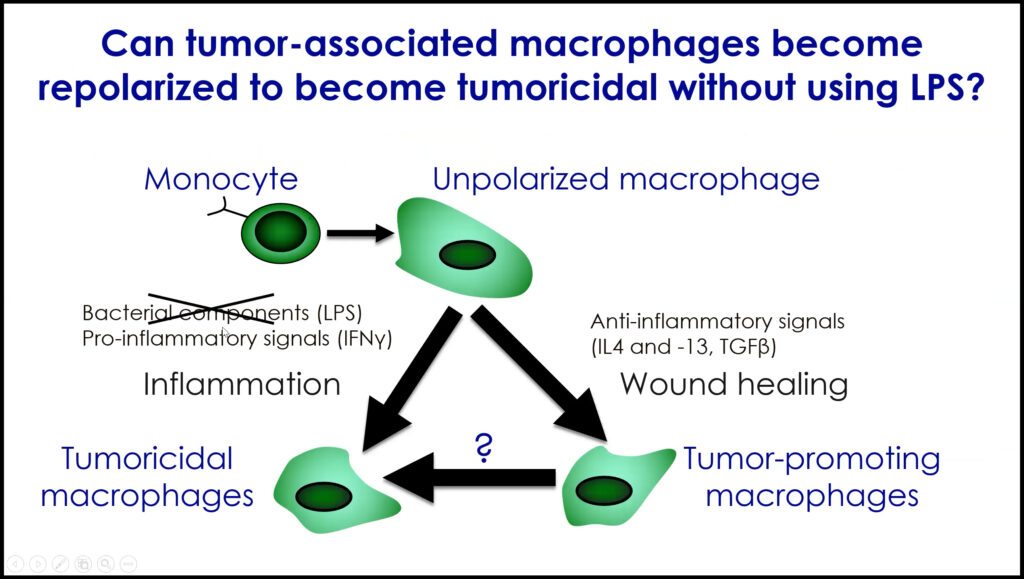
They hypothesized that if they delivered MPLA and IFNγ to tumors, macrophages would become tumoricidal. The lab observed that tumors from both mice and patients with breast cancer contained anti-tumor macrophages following treatment, which stopped metastatic progression. Egeblad and her team plan to elucidate how long lasting the responses are and whether they can be further improved by combining this treatment with checkpoint blockade immunotherapy.
Further Readings
Sosman
Johnson DB, Balko JM, Compton ML, et al
Fulminant Myocarditis with Combination Immune Checkpoint Blockade
N Engl J Med. 2016;375(18):1749-1755
Postow MA, Sidlow R, Hellmann MD
Immune-Related Adverse Events Associated with Immune Checkpoint Blockade
N Engl J Med. 2018;378(2):158-168
Puzanov I, Diab A, Abdallah K, et al
J Immunother Cancer. 2017;5(1):95
Pallin DJ, Baugh CW, Postow MA, Caterino JM, Erickson TB, Lyman GH
Immune-related Adverse Events in Cancer Patients
Acad Emerg Med. 2018;25(7):819-827
Weber JS, Kähler KC, Hauschild A
Management of immune-related adverse events and kinetics of response with ipilimumab
J Clin Oncol. 2012;30(21):2691-2697
Weber JS, Yang JC, Atkins MB, Disis ML
Toxicities of immunotherapy for the practitioner
J Clin Oncol. 2015;33(18):2092-2099
Egeblad
Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al
Targeting potential drivers of COVID-19: Neutrophil extracellular traps
J Exp Med. 2020;217(6).
Zuo Y, Yalavarthi S, Shi H, et al
Neutrophil extracellular traps in COVID-19
JCI Insight. 2020;5(11)
Kolaczkowska E, Kubes P
Neutrophil recruitment and function in health and inflammation
Nat Rev Immunol. 2013;13(3):159-175
Engelhardt JJ, Boldajipour B, Beemiller P, et al
Cancer Cell. 2012;21(3):402-417
Albrengues J, Shields MA, Ng D, et al
traps produced during inflammation awaken dormant cancer cells in mice
Science. 2018;361(6409)
Immunopathology and Engineered Immune Cells
Speakers
Crystal Mackall, MD
Stanford University
Garry Nolan, PhD
Stanford University
Pathology from the Molecular Scale on Up
Garry Nolan has developed multiple technologies to improve our understanding of both normal immune function and immunopathologies. One such tool termed CODEX (CO-Detection by indexing) modifies traditional fluorescent microscopes for high-dimensional imaging so that users can obtain spatial and quantitative insight into cells within complex tissues. As a multiplexed imaging platform, CODEX allows users to stain tissue with 50-120 antibodies that have unique barcodes that can be annealed to fluorophores for visualization. Interestingly, Nolan’s lab has used this technology to identify specific “neighborhoods” of immune cells, defined as tissue regions within each cell that have a similar surrounding of cell types. They have also discovered that cellular neighborhoods communicate with each other and can change during various stages of cancer. This information provides critical insight into disease progression that may inform clinical outcomes in the future.
Next Generation CAR T Cells
Crystal Mackall studies the basis of tumor-immune interactions to develop new cancer immunotherapies. Specifically, her lab works to improve chimeric antigen receptor T cell (CAR T cell) therapy, in which a patient’s T cells are engineered to express a synthetic receptor that recognizes an antigen on their tumor. Despite its effectiveness, some patients become unresponsive to this treatment over time. “As we think about the future of this field, we need to talk about the issue of resistance,” said Mackall, “we need to unpack it and identify where and how these cells are failing.” The loss of specific antigen expression has been identified as a significant contributor to resistance, as it can eliminate the ability of CAR T cells to recognize the tumor.
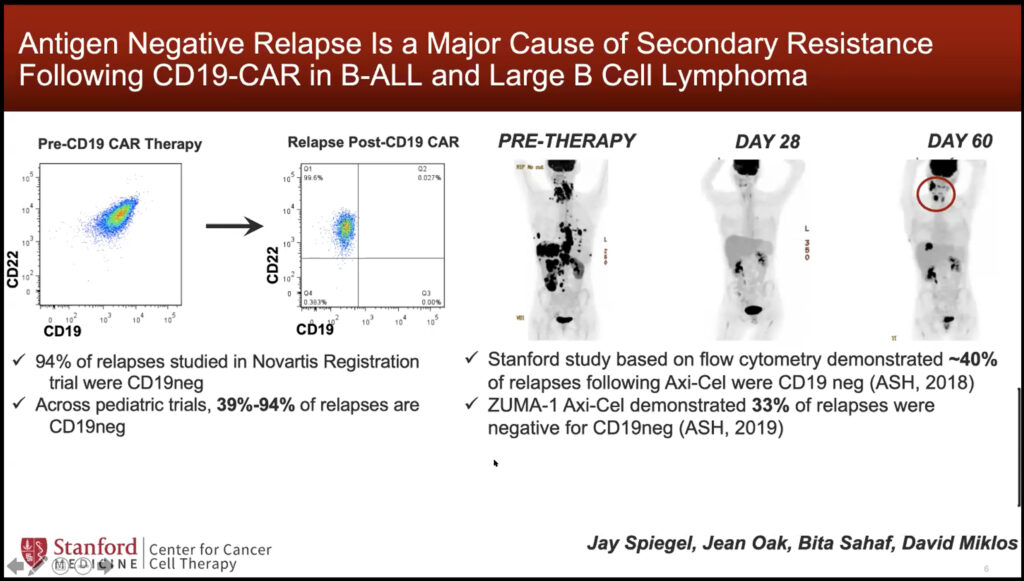
To address this problem, Mackall’s lab engineered a “multi-specific CAR” to simultaneously target two antigens. Even if one antigen’s expression is lost over time, the other will still be targeted which may reduce resistance. In a clinical trial, she found that this therapeutic strategy was well tolerated in both children and adults with leukemia and lymphoma. Mackall’s lab will further optimize the engineering of other multi-specific CARs to overcome resistance.
Further Readings
Nolan
Lee IT, Nakayama T, Wu C-T, et al
medRxiv. May 2020
Lu S, Stein JE, Rimm DL, et al
JAMA Oncol. July 2019
Goltsev Y, Samusik N, Kennedy-Darling J, et al
Deep Profiling of Mouse Splenic Architecture with CODEX Multiplexed Imaging
Cell. 2018;174(4):968-981.e15
Mackall
Fry TJ, Shah NN, Orentas RJ, et al
Nat Med. 2018;24(1):20-28
Majzner RG, Mackall CL
Clinical lessons learned from the first leg of the CAR T cell journey
Nat Med. 2019;25(9):1341-1355
Locke FL, Ghobadi A, Jacobson CA, et al
Lancet Oncol. 2019;20(1):31-42
Neelapu SS, Locke FL, Bartlett NL, et al
Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma
N Engl J Med. 2017;377(26):2531-2544
Maude SL, Laetsch TW, Buechner J, et al
Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia
N Engl J Med. 2018;378(5):439-448