Maternal, Neonatal, and Early Infancy Vaccine Developments
Reported by:
Mercedes Vega Villar
Presented by:
Microbiology and Infectious Diseases Discussion Group
The New York Academy of Sciences
Overview
Newborns have a limited ability to fight infectious pathogens. As a result, morbidity and mortality are high during the first few months of life, particularly in low- and middle-income countries. Active research is taking place, and great strides have been made to identify the factors that influence the risk of infection and disease in pregnant women and newborns. One promising approach—maternal, neonatal, and early infancy vaccines—has the potential to protect the health of mothers and children worldwide. Although several existing vaccines are safe and effective for use during pregnancy, no vaccines have been formally approved or licensed specifically for use in pregnant women.
On June 23, 2020, the New York Academy of Sciences hosted a symposium to explore challenges and achievements in the development and licensure of maternal vaccines. In a series of keynote and plenary presentations, the symposium covered recent findings regarding the maternal-to-newborn transfer of antibodies, lessons learned from the use of licensed vaccines during pregnancy, new targets for maternal immunization and contextual factors that influence the vaccination of pregnant women and their offspring.
Symposium Highlights:
- Infectious disease is one of the leading causes of stillbirth and newborn death around the world.
- Because antibodies can be transferred from the mother to the baby, boosting maternal antibodies can help protect the newborn against respiratory or invasive diseases.
- Certain vaccines that are routinely used in the general population (e.g., influenza, pertussis), are also recommended for pregnant women to protect both mother and child.
- Several pregnancy-specific vaccines, such as Group B Streptococcus, Respiratory Syncytial Virus, and Human Cytomegalovirus, are currently in different stages of development.
- Contextual factors, like access to health care during pregnancy and after birth, are important determinants in the success of maternal and neonatal immunization worldwide.
Speakers
Stephen L. Brusatte
The University of Edinburgh
Sinéad Farrington
The University of Edinburgh
John Marioni
European Bioinformatics Institute and University of Cambridge
David P. Mills
The University of Manchester
Artem Mishchenko
The University of Manchester
Matthew Powner
University College London
Themis Prodromakis
University of Southampton
Edze Westra
University of Exeter
Sponsors
Promotional Partners
Keynote: Protecting Infants with Maternal Vaccination
Speaker
Shabir Madhi
University Witwatersrand
Prospects of Maternal Vaccination to Protect Young Infants
One of the United Nations’ Sustainable Development Goals is to reduce the global maternal mortality rate and eradicate preventable deaths in newborns and children under five by 2030. “Of all children under the age of five who die, roughly two thirds will in fact die within the first six months of life” explained vaccinologist Shabir Madhi. Hence, shielding newborns can really advance the fight against child mortality. Maternal and neonatal vaccines could prevent lethal infections at this vulnerable stage of life. However, to develop those vaccines, we need to understand the biological causes of early-life mortality.
Unfortunately, the quality of epidemiological and clinical data regarding child mortality is poor. Neonatal and fetal deaths are underreported and under-investigated, particularly in countries with the highest rates of early-life mortality. In low- and middle-income countries, autopsies of children are rare. As a result, our understanding of early-life mortality is still limited.
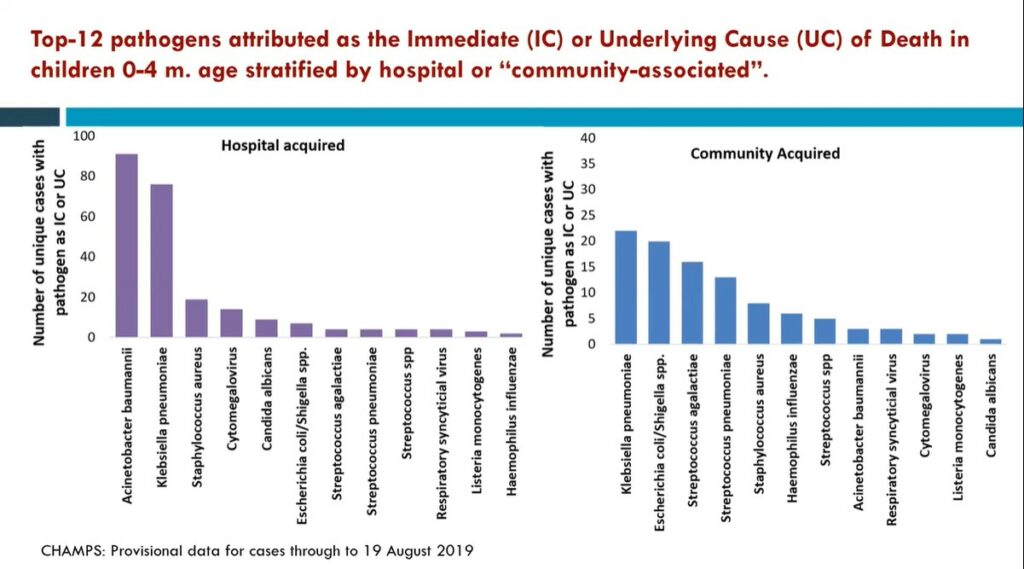
Madhi and his team are trying to fill this research gap. In a large study, Madhi and his colleagues collected tissue samples and clinical records of deceased children across seven countries in Africa and Southeast Asia. This diagnostic approach was sufficient to establish the chain of factors that may have ultimately caused the deaths of these infants. Madhi and his team also sought to ascertain the biological causes of stillbirth. Together, these studies revealed that infections accounted for about 20% of stillbirths and 54% of newborn deaths. Furthermore, the investigators were able to identify the top pathogens behind those infections, like K. pneumoniae, or E. coli. These findings can help inform the strategic development of maternal vaccines, a promising intervention in the fight against child mortality.
Further Readings
Madhi
Saha SK, Schrag SJ, El Arifeen S, et al
The Lancet. 2018 Jul 6; 392 (10142):145-159.
Taylor AW, Blau DM, Bassat Q, et al
The Lancet Global Health. 2020 Jul;8(7):e909-e919
Nunes MC, Aquil AR, Omer SB, Madhi S
Am J of Perinatol. 2016 Sept;33(11): 1104-1114
Madhi S, Briner C, Maswime S, Mose S
Causes of stillbirths among women from South Africa: a prospective, observational study
The Lancet Global Health. 2019 Apr; 7(4):e503-e512
Madhi S, Polack FP, Piedra PA, et al
Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants
N Eng J Med. 2020 Jul 30;383(5):426-439
Maternal-Fetal Immunology and Physiology
Pat Furlong, Panelist
Parent Project Muscular Distrophy
Roman J. Giger
University of Michigan School of Medicine
Altered Mother-To-Newborn Transmission of Pioneering Microbiota and Child Health
The human body is host to a large number of microbes, such as bacteria and fungi. This community of microorganisms, or microbiota, occupies the gut, skin, and cavities. It plays a role in essential functions, like the promotion of immune responses and the production of vitamins. So it’s not surprising that changes to the composition of the microbiota are associated with various diseases. In his research, epidemiologist Noel Mueller studies how the gut microbiota affects metabolic health during early development.
The first exposure to these microbes occurs in the birth canal. This mom-to-newborn transfer of microbiota provides the baby with a healthy collection of microorganisms. Mueller and his team have found that babies delivered by cesarean section (CS) have less of these protective microbes. Instead, opportunistic pathogens are more likely to colonize these babies’ microbiota. The proportion of cesarean-delivered babies continues to increase worldwide. As a result, “even if [CS] poses a small relative risk in health outcomes, it can have a large impact at the population level,” Mueller explained.
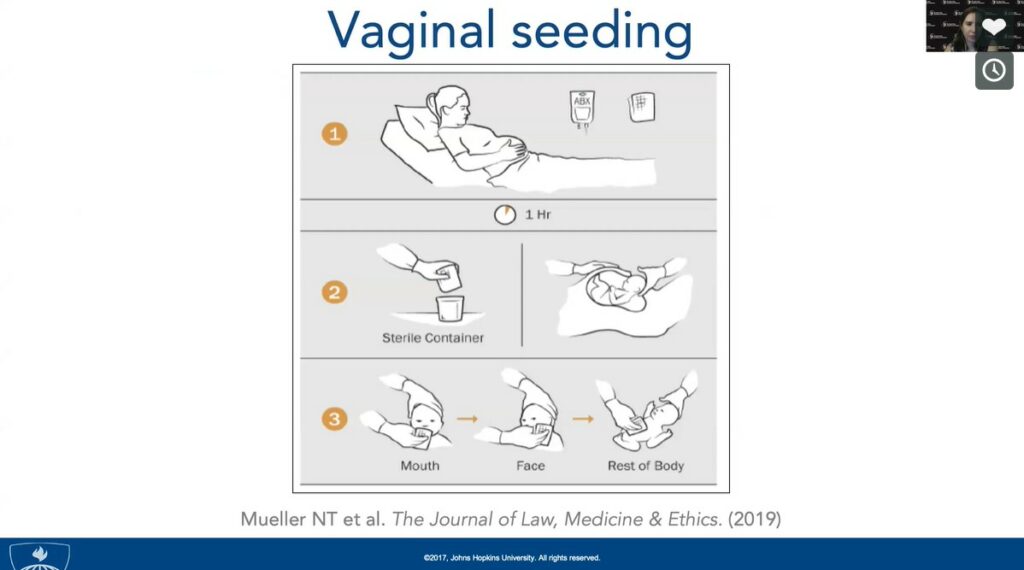
CS is associated with a higher risk of developing allergies, obesity, and asthma. Lack of exposure to the mother’s microbiota in the birth canal may be at the root of some of these disorders. Mueller and his collaborators investigated a simple intervention called “vaginal seeding,” which consists of inoculating cesarean-delivered babies with their mother’s microbiota. In this “bacterial baptism,” maternal vaginal microbiota is wiped in the neonate’s face, mouth, and body. Preliminary data suggest that vaginal seeding can restore some beneficial microbes that may otherwise be lacking in these babies. A large randomized controlled trial assessing the efficiency and safety of this technique is currently underway.
Antibody Transfer From Mother To Infant: The Physiology of Breast Milk
For Kirsty Le Doare, an expert in pediatric infectious disease, breast milk is like your grandmother’s chicken soup. “It contains all the nutrients that a baby needs to grow and thrive,” she said. In addition, breast milk also protects infants from infections. Hence, breastfed infants are less vulnerable to gastrointestinal and respiratory tract infections than formula-fed infants. However, it is still unclear how breast milk modulates the infant immune response.
Le Doare wants to change that through her research on age-related immune response to infectious diseases. She, along with her team, conducted a study with Gambian mother/infant pairs. They found that IgA antibodies for Group B Streptococcus (GBS) in breast milk reduced the likelihood of GBS colonization days after birth and increased the likelihood of GBS clearance later in life. Interventions like maternal vaccines can harness this form of neonatal protection against pathogens.
After some forms of maternal immunization, antibodies against certain pathogens persist in the breast milk for at least six months, protecting the neonate. Le Doare and her team are using novel culture models to understand how these antibodies fight pathogens. Although breast milk antibodies are a critical protective agent for the neonate, oligosaccharides in breast milk have also been found to lower the risk of infection. For instance, these sugar molecules can act as a decoy for pathogens, which bind to them and stay away from intestinal cells. A better understanding of these mechanisms could be leveraged to develop therapies to protect neonates against invasive diseases.
The Structure of the Human Placenta Enables Humoral and Cellular Immune Defense
According to virologist Lenore Pereira, 1%-3% of infants are infected with Human Cytomegalovirus (HCMV) every year. In about 25% of infected babies, HCMV can cause congenital disorders such as cerebral palsy, microcephaly, and deafness. Mothers that have strong neutralizing antibodies due to prior HCMV infection are less likely to transmit the infection to the baby, and most infants born of seropositive mothers are asymptomatic. However, the immune mechanisms that help prevent infection are not well understood.
HCMV infects the developing baby by crossing the uterine-placental interface and replicating in the placenta. Using ex vivo explants of first-trimester placentas, Pereira and her team have been able to identify the mechanisms of infection as well as the protective potential of some antibodies. Neutralizing antibodies, namely those that target a specific complex of glycoproteins in the surface of HCMV, reduce viral spread, infection, and cell death in early-gestation placentas.
The investigators also demonstrated that, in the maternal component of the placenta, HCMV infection induces an increase in effector-memory T cells. These cells may confer protection to the babies of seropositive mothers. These findings could lead to the development of antibody treatments that restrict HCMV infection and associated pregnancy complications from congenital infection.
Further Readings
Mueller
Mueller NT, Bakacs E, Combellick J, et al
The infant microbiome development: mom matters
Trends Mol Med. 2015 Feb;21(2):109-17
Mueller NT, Shin H, Pizoni A, et al
Scientific Reports. 2016 Apr 1;6(23133)
Shao Y, Forster SC, Tsaliki E, et al
Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth
Nature. 2019;574(7776):117-121
Mueller NT, Hourigan SK, Hoffman DE, et al
Journal Law Med Ethics. 2019;47(4): 568-578
Le Doare
Le Doare K, Faal A, Jaiteh M, et al
Vaccine. 2017 May 19;35(22):2970-2978
Andreas NJ, Al-Khalidi A, Jaiteh M, et al
Role of human milk oligosaccharides in Group B Streptococcus colonisation
Clin Transl Immunology. 2016 Aug 26; 5(8):e99
Le Doare K, Holder B, Bassett A, Pannaraj PS
Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity
Front Immunol. 2018 Feb 28;9(361)
Pereira
Tabata T, Pettit M, Fang-Hoover J, et al
Vaccines. 2019 Dec;7(4):135
Pereira L
Congenital Viral Infection: Traversing the Uterine-Placental Interface
Annu Rev Virol. 2018 Sep 29;5(1):273-299
Maidji E, Genbacev O, Chang H, Pereira L
Journal of Virology. 2007 June;81(9):4701-4712
Pereira L, Maidji E, McDonagh S, Tabata T
Insights into viral transmission at the uterine-placental interface
Trends Microbiol. 2005;13(4):164-174
Lessons from Current Vaccines & New Targets for Immunization
Speakers
Janet Englund, MD
Seattle Children’s Hospital
Stanley Plotkin, MD
Vaxconsult LLC, University of Pennsylvania
Ajoke Sobanjo-ter Meulen, MD, MSc
Bill & Melinda Gates Foundation
Maternal Vaccines Licensed for Adults: What Can Be Learned from Recent Experiences with Influenza, Pertussis and Tetanus Vaccines
Maternal immunization is not a new strategy, said pediatrician and infectious disease expert, Janet Englund. Englund discussed the history of maternal vaccines, whose popularity has ebbed and flowed throughout the years. As early as 1879, maternal immunization was used to confer protection from smallpox in infants.
For centuries, tetanus toxoid was one of the leading causes of neonatal death. A landmark study in New Guinea demonstrated that maternal immunization with tetanus toxoid protects newborns. As a result, this vaccine has been routinely administered to pregnant women for years. Thanks to this, Englund said, 45 countries have been able to eradicate maternal and neonatal tetanus, a testament to the potential of maternal immunization to fight global epidemics.
In 2012, an outbreak of pertussis was observed in the US and other developed countries, including the UK. In October of that year, the UK introduced the maternal pertussis vaccine, which was associated with a dramatic decrease in the rate of neonatal pertussis. While most health authorities recommend that pregnant women get this vaccine during the third trimester, recent studies have shown that earlier is better; maternal immunization during the second trimester maximizes pertussis antibody transfer to the infant.
Pregnant women, Englund explained, are at a higher risk of severe influenza infections than non-pregnant women. Influenza infection during pregnancy is associated with preterm birth and low birth weight infants. Maternal influenza immunization is safe and it effectively protects the mother and the newborn. However, less than 50% of pregnant mothers in the US get vaccinated. Along with issues of insufficient insurance coverage, “vaccine hesitancy plays a very important role”, Englund lamented.
Human Cytomegalovirus Vaccines
Physician and vaccinologist Stanley Plotkin gave another presentation on cytomegalovirus (CMV). While co-speaker Lenore Pereira focused on the mechanisms of transplacental CMV transmission, Plotkin looked at the factors involved in CMV infection during pregnancy, immunity, and the status of CMV vaccine development status.
Many people, particularly in developing countries, become infected with cytomegalovirus (CMV) at some point in their lives. CMV infection does not cause obvious disease in most healthy individuals. However, in pregnant women, CMV can cross the placenta and infect the fetus. This can lead to congenital disorders such as microcephaly or deafness. CMV infection is also more serious for adults with weakened immune systems.
Plotkin explained that CMV infection during pregnancy usually occurs when mothers are exposed to toddlers carrying the virus. In developing countries, CMV infects most women before they become pregnant, and newborns are less likely to suffer from congenital disorders upon their mothers’ CMV infection. “We think that natural immunity is protective,” said Plotkin. In a pioneering study, his team found that vaccinating kidney transplant recipients with a weak strain of CMV protected them from contracting severe disease.
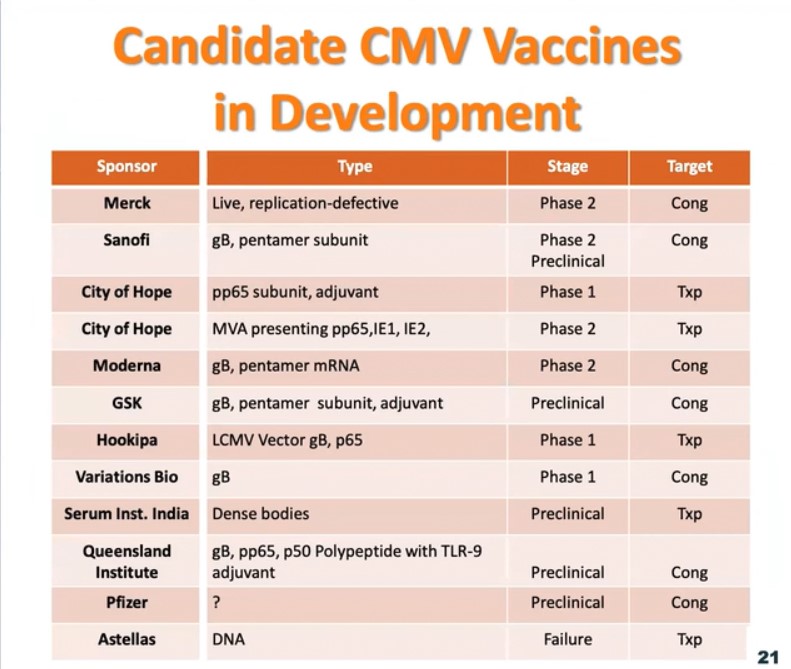
One of the most promising vaccine candidates is the glycoprotein-B (gB) with MF59 adjuvant. This vaccine induces a strong antibody response, and these antibodies protect against CMV infection. Other CMV vaccines are also in the research and development phase. As these vaccines become approved and licensed, some of the first populations to be vaccinated will probably be transplant patients, young girls, and women of child-bearing age who have not been previously infected by CMV.
Updates on Group B Streptococcus (GBS) Vaccine Development
Ajoke Sobanjo-ter Meulen, infectious disease and global health expert, shed light on the current state of Group B Streptococcus (GBS) vaccine development. GBS is a pathogen commonly found in the gut or lower vaginal tract. Because it can cause life-threatening infections in pregnant women, newborns, and immunocompromised adults, it is associated with maternal and neonatal morbidity and mortality, preterm births, and stillbirths. Fatality rates due to GBS infection are higher in low- and middle-income countries.
If pregnant women in the US test positive for GBS colonization, they are treated with antibiotic prophylaxis, which significantly reduces disease during the first hours of life. However, a vaccine has a higher protective potential and it would be a much more feasible approach in low- and middle- income countries, where the diagnostic and therapeutic infrastructure required for that kind of treatment may not be available.
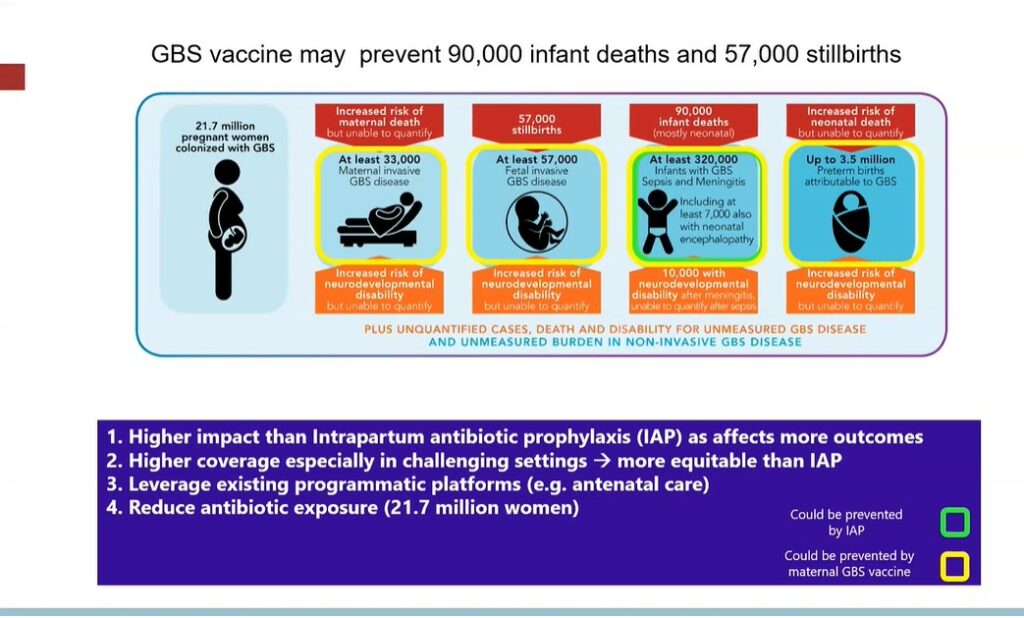
GBS vaccines given to women during pregnancy can significantly reduce maternal GBS colonization and disease, stillbirths and neonatal sepsis, and meningitis. In a modelling study, Sobanjo-ter Meulen and colleagues estimated that, by improving all of these health outcomes, a maternal GBS vaccine could prevent 90,000 infant deaths and 57,000 stillbirths worldwide. Several vaccine candidates are currently being developed. One challenge facing licensure trials is that, due to the low incidence, a sample size of about 40,000-60,000 pregnant women would be required to ascertain the efficacy of a maternal GBS vaccine.
According to Sobanjo-ter Meulen, public oversight and private investment could help overcome these challenges. That kind of partnership has helped advance the GBS6 vaccine, which demonstrated high safety and immunogenicity levels in healthy adults and is currently being evaluated in a phase-II trial in pregnant women in South Africa.
Further Readings
Englund
Steinhoff MC, Katz J, Englund JA, et al
Lancet Infect Dis. 2017 Sep;17(9):981-989
Kozuki N, Katz J, Englund JA, et al
Int J of Gynaecol and Obstet. 2018 Jan;140(1):65-72
Englund JA
Maternal immunization with inactivated influenza vaccine: rationale and experience
Vaccine. 2003 Jul 28;21(24):3460-3464
Plotkin
Plotkin SA, et al
The Status of Vaccine Development Against the Human Cytomegalovirus
J Infect Diseases. 2020 Mar 5;221(Supplement_1):S113-S122
Manicklal S, Emery VC, Lazzarotto T, et al
The “silent” global burden of congenital cytomegalovirus
Clin Microbiol Rev. 2013 Jan;26(1):86-102
Bernstein DI, Munoz FM, Callahan ST, et al
Vaccine. 2016 Jan 12;34(3):313-319
Sobanjo-ter Meulen
Seale AC, Baker CJ, Berkley JA, et al
Vaccine. 2019 Aug 14;37(35):4877-4885
Vekemans J, Moorthy V, Friede M, et al
Vaccine. 2019 Nov 28;37(50):7391-7393
Seale AC, Bianchi-Jassir F, Russell NJ, et al
Clin infect Dis. 2017 Nov 6;65(suppl_2):S200-S219
Sobanjo-Ter Meulen A, Abramson J, Mason E, et al
Vaccine. 2015 Nov 25;33(47):6388-6395
Infant Vaccination and Barriers to Maternal Immunization
Speakers
Barney Graham, MD, PhD
National Institute of Allergy and Infectious Diseases (NIAID), NIH
Ruth Karron, MD
Johns Hopkins Bloomberg School of Public Health
Approaches to Infant Vaccination for Respiratory Syncytial Virus
The Respiratory Syncytial Virus (RSV) is a common cause of respiratory tract infections. In newborns, RSV infection can lead to severe disease, usually due to obstruction of small airways. One of the key goals in RSV vaccine development, said immunologist and virologist Barney Graham, is to protect children younger than six months of age from RSV infection.
A failed vaccine trial in the 1960s stalled RSV vaccine development. In the study, vaccinated children became more vulnerable to RSV infection and were more likely to experience severe illness than control groups. According to Graham, the field of RSV vaccine development has only regained speed within the last 10-15 years.
Graham’s work in the structure of RSV fusion protein (F) has been critical in recent advances in RSV vaccine development. This glycoprotein on the surface of RSV mediates viral entry into host cells. During cell entry, the structure of F proteins undergoes a conformational change. The prefusion and postfusion forms of F proteins have different properties as vaccine antigens. Graham and his collaborators led a phase-I clinical trial using a stabilized prefusion F protein. In young adults, this vaccine elicited a potent neutralizing activity, and it is currently being tested in phase-II maternal immunization trials.
A similar strategy can be generalized across other virus families, including coronaviruses. In fact, his work in structure-based immunogen design allowed Graham and colleagues to rapidly solve the structure of the new coronavirus and has greatly contributed to the current efforts to develop a candidate vaccine.
Maternal Immunization: Overcoming Barriers to Uptake and Access
Vaccine research and development is not the only obstacle in maternal and neonatal immunization. “In the end, vaccines are only useful if they can be delivered,” said global health expert Ruth Karron, before discussing contextual factors that influence the successful implementation of maternal vaccines.
When it comes to pregnancy-specific vaccines, medical, social, and economic considerations are paramount. These vaccines are usually administered in the context of antenatal care. Without a robust antenatal care system, implementing an effective maternal immunization program can be challenging. For instance, vaccines that can be administered with some flexibility throughout the second or third trimester will be more likely to lead to successful outcomes in cases when pregnant women have limited opportunities to go to the doctor. Also, attitudes surrounding maternal immunization need to be considered. Vaccine hesitancy can be particularly acute when it comes to pregnancy, as some believe it can be harmful for the child or negatively affect fertility in the future. Failing to understand the effects or the burden of a specific disease can also be an obstacle. These issues have to be addressed broadly with all stakeholders, which include the pregnant women but also other community members, healthcare providers, and policymakers.
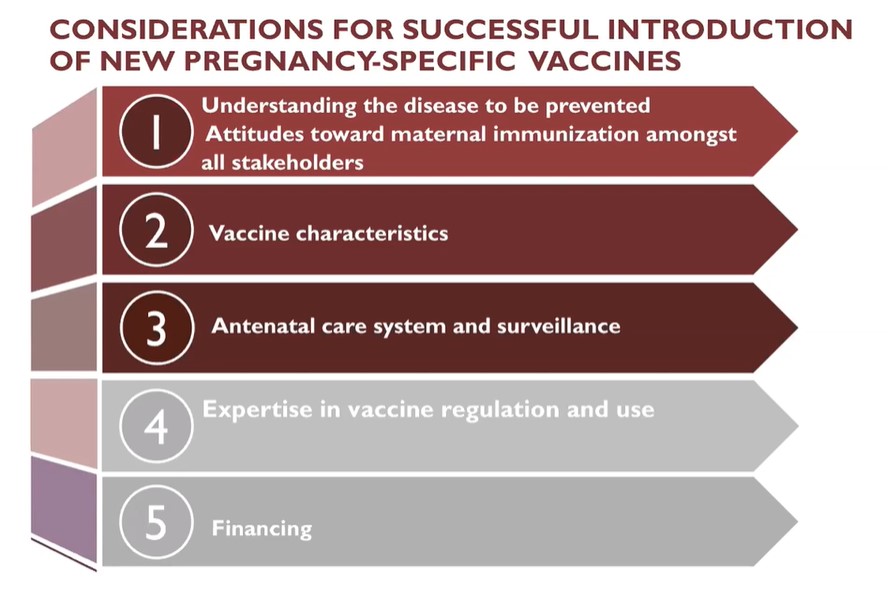
Karron and her collaborators created ethical guidelines to include the interests of pregnant women and their offspring in the development of vaccines . Some of these guidelines are very relevant in the context of epidemics. As the current COVID-19 pandemic has revealed, pregnant women and their offspring are among the most severely affected by outbreaks. They are more vulnerable to infections, but they are also disproportionately affected by an overburdened health care system. In her work, Karron advocates for the inclusion of women of childbearing age and pregnant women at every stage of vaccine development and deployment.
Further Readings
Graham
Johnson JE, Gonzales RA, Olson SJ, et al
The histopathology of fatal untreated human respiratory syncytial virus infection
Mod Pathol. 2007 Jan;20(1)108-19
Graham BS, Gilman MSA, McLellan JS, et al
Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody
Science. 2013 May 31;340(6136):1113-1117
Corbett KS, Edwards DK, Leist SR, et al
SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness
Nature. 2020 Aug 5
Karron
Giles ML, Mason EM, Lambach P, Mantel C
Maternal immunization country readiness: a checklist approach
Hum Vacc Immunother. 2020 May 27;1(7).
Krubiner CB, Faden RR, Karron R, et al.
Vaccine. 2019 May 3;S0264-410X(19):30045-3

