New Developments in Human Healthspan and Longevity
Reported by:
Adele Whaley
Presented by:
American Federation for Aging Research
Aspen Brain Institute
Science Translational Medicine
The New York Academy of Sciences
Overview
Although advances made in health and safety have more than doubled life expectancy throughout much of the world since 1900, it hasn’t been without consequence. Disease, disability, and frailty have all impacted the quality of life associated with these later years. This unfortunate reality was recently illuminated by the COVID-19 pandemic, which severely affected this population, likely due to physiological changes and preexisting conditions. Fortunately, a primary goal of geroscience researchers is to attenuate age-related health issues so that older people not only enjoy an improved quality of life, but also maintain the resilience to survive severe diseases and infections.
While it’s irrefutable that we cannot avoid aging, it’s no longer within the realm of science fiction for us to temper and even reverse the aging process. On May 19, 2021, the New York Academy of Sciences hosted a virtual symposium that brought together geroscience experts spanning various disciplines, including genetics, endocrinology, gerontology, clinical psychology, and more. Speakers discussed targeting the key hallmarks of aging, developing biomarkers for geriatric therapies, and translating findings that extend healthspan and lifespan to the clinic.
Symposium Highlights
- The Target Aging with Metformin study uses the FDA approved anti-diabetic metformin, which targets the hallmarks of aging, to investigate the prevention of age-related diseases.
- Precluding the age-associated decline of chaperon-mediated autophagy restrains the aggregating effects of Alzheimer’s disease and extends lifespan in murine models.
- Lower IGF-1 levels in older adults are associated with decreased cognitive impairment, age-related diseases, and mortality.
- Epigenetic clocks can be applied to study biological aging differences, with accelerated epigenetic aging correlating with the prevalence and incidence of morbidity and mortality.
- The metabolome is a powerful locus of opportunity to bridge the gap between genotype and age.
- Alternative splicing is upregulated in response to declining mitochondrial function and increasing age.
- Senescent cells upregulate pro-survival pathways, and their elimination alleviates diverse age-related conditions.
- The mitochondrial-derived peptides humanin and MOTS-c are associated with increased longevity in animal models and humans.
Speakers
Nir Barzilai, MD
Albert Einstein College of Medicine
Ana Maria Cuervo, MD, PhD
Albert Einstein College of Medicine
Sofiya Milman, MD
Albert Einstein College of Medicine
Morgan Levine, PhD
Yale School of Medicine
Daniel Promislow, PhD
University of Washington
Luigi Ferrucci, MD, PhD
National Institute on Aging, National Institutes of Health
James Kirkland, MD, PhD
Mayo Clinic
Pinchas Cohen, MD
USC Leonard Davis School of Gerontology
Targetable Aging Processes
Speakers
Nir Barzilai, MD
Albert Einstein College of Medicine
Ana Maria Cuervo, MD, PhD
Albert Einstein College of Medicine
Keynote: Age Later: Translational Geroscience
Aging is the strongest risk factor for all age-related diseases, with diverse maladies accumulating during the later years of life. Hence, to abate or avert the relevant disorders, it’s critical to target the central driver—aging itself. Physician Nir Barzilai, the founding director of the Institute for Aging Research, investigates the genetics of longevity by studying centenarians and their offspring, interrogating the hypothesis that these individuals have genes that prolong aging and protect against age-related diseases.
Using Slow Off-Rate Modified Aptamer, Barzilai’s team assessed 5,000 proteins in a population of 1,000 individuals between the ages of 65-95, a period during which aging accelerates. Results demonstrated a significant change in the level of hundreds of proteins as a function of age. Among the top hits were proteins from collagen breakdown of tissue and cellular products, highlighting the pivotal role this process plays in aging, and suggesting that deterring disintegration may be a universal biomarker for geroprotection.
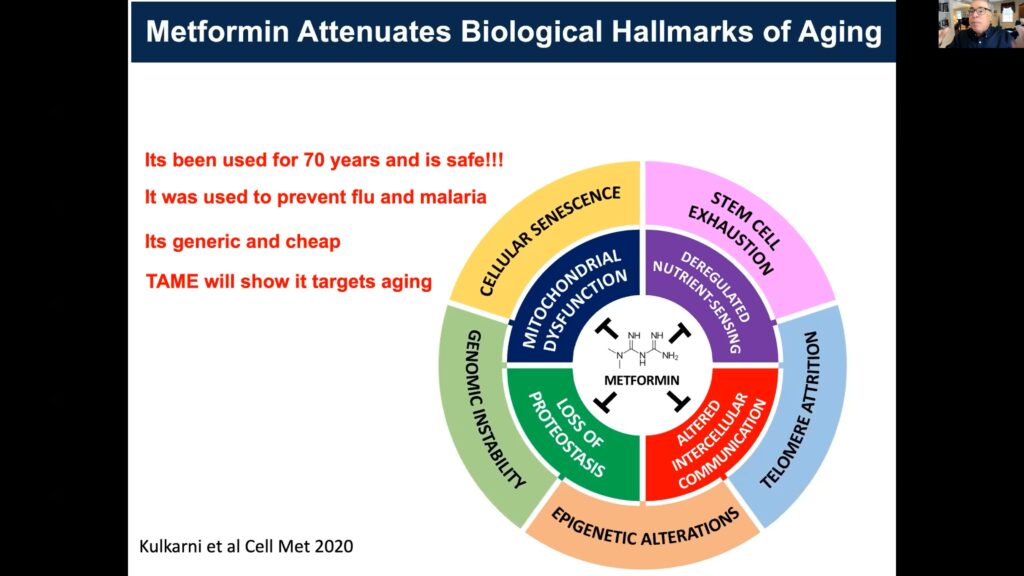
A predominant challenge to translating advances made in geroscience from animal models to humans is the FDA, which currently doesn’t consider aging a disease indication or preventable condition. Barzilai and others are utilizing metformin, an FDA-approved anti-diabetic, to refute this contention. Various groups have shown that metformin has substantial effects on human healthspan, including delaying type-2 diabetes mellitus (T2DM). In this patient subset, metformin also impedes cardiovascular disease, cognitive decline, and Alzheimer’s and is associated with decreased cancer incidence, with population effects approaching 30% in all cases.
Barzilai’s team designed the Target Aging with Metformin, or TAME, study to investigate whether or not there’s a shift in the timeline of disease occurrence between a cohort receiving metformin versus a control cohort. Various biomarkers of aging and age-related diseases will be used to provide convergent evidence of broad, age-related effects, while also establishing a resource for innovation and discovery of emergent biomarkers.
“The most important thing for us is to develop biomarkers that will change when we use a gerotherapuetic,” Barzilai asserted, as this will expedite therapeutic prospects.
Targeting Selective Autophagy in Aging and Age-related Diseases
Physician-scientist Ana Maria Cuervo’s research seeks to understand the molecular basis of autophagy dysfunction with age and the contribution of defects in this cellular pathway to diseases such as neurodegeneration, metabolic disorders, and cancer. Autophagy belongs to the proteostasis network, which regulates protein content and quality control.
Chaperon-mediated autophagy (CMA) is a subset of the mammalian autophagy program that directly targets proteins to the lysosome for degradation. CMA has been shown to decrease with age in human and animal models. Cuervo’s lab developed a fluorescent murine reporter construct to visualize CMA and track the kinetics of its activity in different organs.
Blocking this pathway in neurons resulted in the aggregation of proteins like α-synuclein (α-syn), tau, and others that are causal in Alzheimer’s Disease (AD). Additionally, CMA reporter mice crossed with a mouse model of AD revealed that CMA activity dramatically decreases in the neurons of AD mice.
Leveraging these findings, Cuervo’s group generated a mouse model to restore CMA activity conditionally. Mice with preserved CMA exhibited an extended median and maximal lifespan compared to controls. Evaluation of the proteostasis network in mice with and without CMA restoration revealed major changes in the proteome. Mice in which CMA was preserved more closely resembled younger animals than their age-matched controls.
“By acting in one of these pathways, we can have an impact in the other hallmarks of aging… because of this interconnection among [them],” Cuervo emphasized.
A compound to selectively activate CMA was developed and tested in an AD model, with results illustrating a reduction in tau pathology and microglial activation in the presence of this agent.
Further Readings
Barzilai
Ismail K, Nussbaum L, Sebastiani P, et al.
Compression of Morbidity Is Observed Across Cohorts with Exceptional Longevity.
J Am Geriatr Soc. 2016 Aug;64(8):1583-91.
Sathyan S, Ayers E, Gao T, et al.
Plasma proteomic profile of age, health span, and all-cause mortality in older adults.
Lehallier B, Gate D, Schaum N, et al.
Undulating changes in human plasma proteome profiles across the lifespan.
Nat Med. 2019 Dec;25(12):1843-1850.
Kulkarni AS, Gubbi S, Barzilai N.
Benefits of Metformin in Attenuating the Hallmarks of Aging.
Cell Metab. 2020 Jul 7;32(1):15-30.
Zhang ZD, Milman S, Lin JR, et al.
Genetics of extreme human longevity to guide drug discovery for healthy ageing.
Cuervo
Kaushik S, Cuervo AM.
Nat Med. 2015 Dec;21(12):1406-1415.
Bourdenx M, Martín-Segura A, Scrivo A, et al.
Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome.
Cell. 2021 May 13;184(10):2696-2714.e25.
Kaushik S, Cuervo AM.
The coming of age chaperone-mediated autophagy.
Nat Rev Mol Cell Biol. 2018 Jun;19(6):365-381.
Dong S, Aguirre-Hernandez C, Scrivo A, et al.
Monitoring spatiotemporal changes in chaperone-mediated autophagy in vivo.
Nat Commun. 2020 Jan 31;11(1):645.
Dong S, Wang Q, Kao Y-R, et al.
Chaperone-mediated autophagy sustains haematopoietic stem-cell function.
Nature. 2021 Mar;591(7848):117-123.
Biomarkers for Therapies
Speakers
Sofiya Milman, MD
Albert Einstein College of Medicine
Morgan Levine, PhD
Yale School of Medicine
Translational Geroscience: Role of IGF-1 in Human Healthspan and Lifespan
Physician Sofiya Milman conducts translational research to uncover the genomic mechanisms regulating the endocrine and metabolic pathways involved in age-related conditions like diabetes, cardiovascular disorders, and Alzheimer’s.
“The goal of geroscience is really to extend healthspan, and not necessarily lifespan,” Milman opened. “What we’re really trying to do is to compress the period of morbidity.”
To discover the biological pathways that allow humans to live long, healthy lives, Milman’s team focused on IGF-1: a reduction of this factor has been consistently shown to extend healthspan and lifespan in models. IGF-1 levels peak during the teenage years before gradually declining. If the reduction of IGF-1 protects from aging, Milman reasoned that lower IGF-1 levels would delay aging and prevent age-related diseases.
Examining a cohort of centenarians expressing lower levels of IGF-1 revealed a 50% reduction in cognitive impairment compared to higher IGF-1 level controls. Genetic studies demonstrated that centenarians were enriched for rare mutations in the IGF-1 receptor that diminished signaling. Additionally, individuals 65+ with low IGF-1 had less cognitive impairment, and delayed onset of cognitive impairment, multi-morbidities, and mortality.
Milman’s team also addressed the link between IGF-1 and age. Younger individuals with lower levels of IGF-1 were at an increased risk for mortality and age-related diseases compared to older individuals, while higher levels of IGF-1 in older adults were associated with increased risk. This suggests that the IGF-1 network aligns with the concept of antagonistic pleiotropy, wherein a factor that’s beneficial to individuals when they’re younger may become harmful when they’re older. It’s advantageous to maintain functionality of proteostasis and resilience as an individual gets older, but IFG-1 inhibits programs involved in these processes.
“So from this, we think it would be wise to maintain IGF-1 levels in youth, but to reduce them with aging,” Milman concluded.
Epigenetic Biomarker of Aging for Lifespan and Healthspan
Biological age is defined by changes or alterations in a living system that renders it more vulnerable to failure and is behind the age-related increase in susceptibility to chronic diseases. Unlike chronological age, it is very difficult to measure because it’s unobservable.
Morgan Levine integrates theories and methods from statistical genetics, computational biology, and mathematical demography to develop biomarkers of aging for humans and animal models. Among this work are efforts to establish systems-level outcome measures of aging to facilitate evaluation for gero-protective interventions.
“There’s some disagreement on how we actually quantify [biological age],” Levine started. “But I would argue that it’s really important to try and do so, because quantifying [this] will really help us in a number of endeavors in the field.”
Levin’s lab is particularly interested in epigenetic aging, as aging drastically remodels the DNA methylation landscape, with widespread increases and decreases as a function of age.
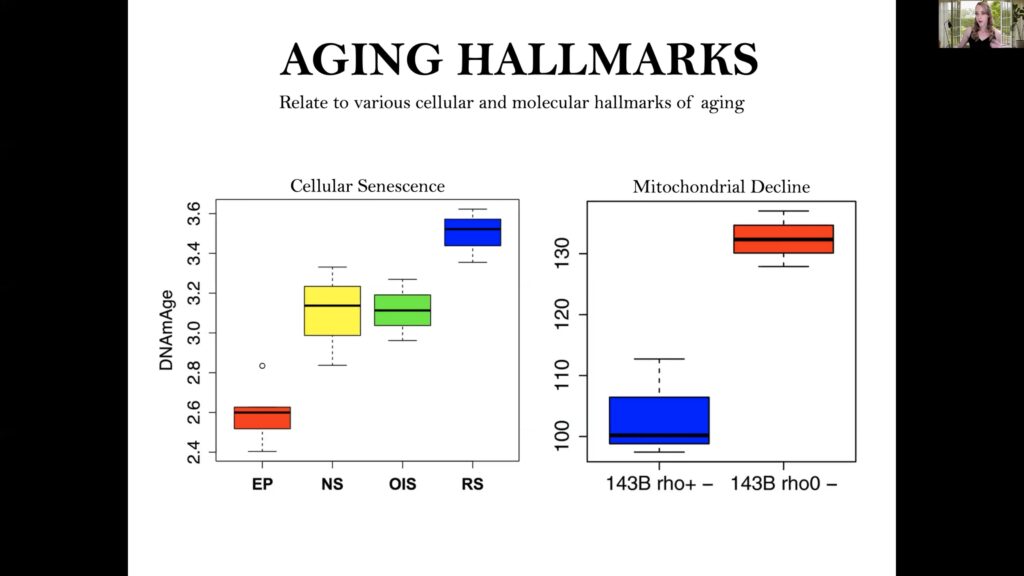
Epigenetic clocks estimate DNA methylation across the genome and combine supervised machine-learning approaches to develop predictors of biological age.
“We think people who have a predicted [epigenetic] age that’s younger than their chronological age should be actually aging slower, whereas the opposite is true for people that have a genetic age that is predicted higher,” said Levine.
Applying these measures to diseased states yielded several pertinent findings. For example, individuals who have pathologically diagnosed Alzheimer’s post-mortem show accelerated epigenetic aging in their brain relative to their chronological age. Tissue differences were also captured, revealing that tissues seem to age asynchronously, with highly proliferative tissues and tumor cells having accelerated aging compared to slower aging brain tissue.
Levine’s group also evaluated cellular senescence and energy disruption, with results revealing that near senescent, HRAS oncogene induced senescent, and replicative stress senescent cells have an acceleration in epigenetic age compared to early parental control cells. Additionally, deletion of mitochondrial DNA accelerated epigenetic aging, while caloric restriction in mice stalled their epigenetic clocks.
Further Readings
Milman
Milman S, Atzmon G, Huffman DM, et al.
Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity.
Aging Cell. 2014 Aug;13(4):769-71.
Zhang WB, Aleksic S, Gao T, et al.
Perice L, Barzilai N, Verghese J, et al.
Aging. 2016 Oct 14;8(10):2414-2424.
Argente J, Chowen JA, Pérez-Jurado LA, et al.
One level up: abnormal proteolytic regulation of IGF activity plays a role in human pathophysiology.
Gubbi S, Quipildor GF, Barzilai N, et al.
40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain.
J Mol Endocrinol. 2018 Jul;61(1):T171-T185.
Levine
Hannum G, Guinney J, Zhao L, et al.
Genome-wide methylation profiles reveal quantitative views of human aging rates.
Mol Cell. 2013 Jan 24;49(2):359-367.
Levine M, McDevitt RA, Meer M, et al.
A rat epigenetic clock recapitulates phenotypic aging and co-localizes with heterochromatin.
Levine ME, Lu AT, Quach A, et al.
An epigenetic biomarker of aging for lifespan and healthspan.
Liu Z, Leung D, Thrush K et al.
Underlying features of epigenetic aging clocks in vivo and in vitro.
Aging Cell. 2020 Oct;19(10):e13229.
Omics for Therapies
Speakers
Daniel Promislow
University of Washington
Luigi Ferrucci
National Institute on Aging, National Institutes of Health
Metabolomics in the Search for Biomarkers and Mechanisms of Aging
Daniel Promislow applies metabolomics and systems biology approaches to study aging, with a focus on understanding the evolutionary and molecular traits that shape fitness in the natural human population. Although genome-wide association studies have allowed researchers to identify thousands of polymorphisms associated with the complement of measurable traits, including aging, the disparities identified explain less than half of 1% of the phenotypic variations.
Many genes interacting with each other ultimately influence phenotypes, and the biological distance between the two is astronomical. To bridge this gap, researchers use endophenotypes—from the epigenome, transcriptome, proteome, metabolome, and microbiome—along with various omics approaches. Promislow’s lab focuses on the metabolome, which integrates information from the environment and genotype to ultimately affect aging.
Promislow’s team utilizes translational metabolomics in various insect and animal models to understand and translate aging patterns to human populations. Applying this approach to Drosophila demonstrated that the metabolome could predict stress resistance, completely separating groups of sensitive or resistant flies to a metabolic stressor by principal component analysis. These effects could not be recapitulated with a whole fly genome sequence dataset. Evaluating response to diet restriction (DR) also revealed changes in metabolite levels with age. Among nearly 200 different inbred strains, roughly 75% showed a benefit to DR.
“Interestingly, the effect of specific genetic variants on the lifespan response was very weak,” Promislow began. “But we did find genes that were associated with metabolites, which were associated with the lifespan response, reinforcing this idea…that the metabolite profile can be a kind of bridge between genotype and phenotype.”
Promislow’s group also demonstrated that the metabolome could serve as a biological clock, revealing that shorter-lived genotypes appeared to have a higher biological age than expected for their chronological age.
Translational Potential of the Biology of Aging
As individuals age, the incidence of chronic disease increase, and disease progression quickens. Physician-scientist Luigi Ferrucci aims to interrogate the causal pathways that lead to progressive physical and cognitive decline in aging.
Cellular damage is accumulated during a person’s life, eventually reaching a pathology threshold that becomes clinically relevant when the damage presents as a disorder. Conventionally, the disease is often only addressed once it reaches this stage. The problem with this approach is that the present disease is often a marker of a more profound and invasive disorder to come.
“[Instead], we need to measure the underlying force that determines the emergence of diseases and their consequences,” Ferrucci argued.
By interfering with the basic mechanisms of aging to curtail it, broader effects of abating multiple chronic disorders can be achieved.
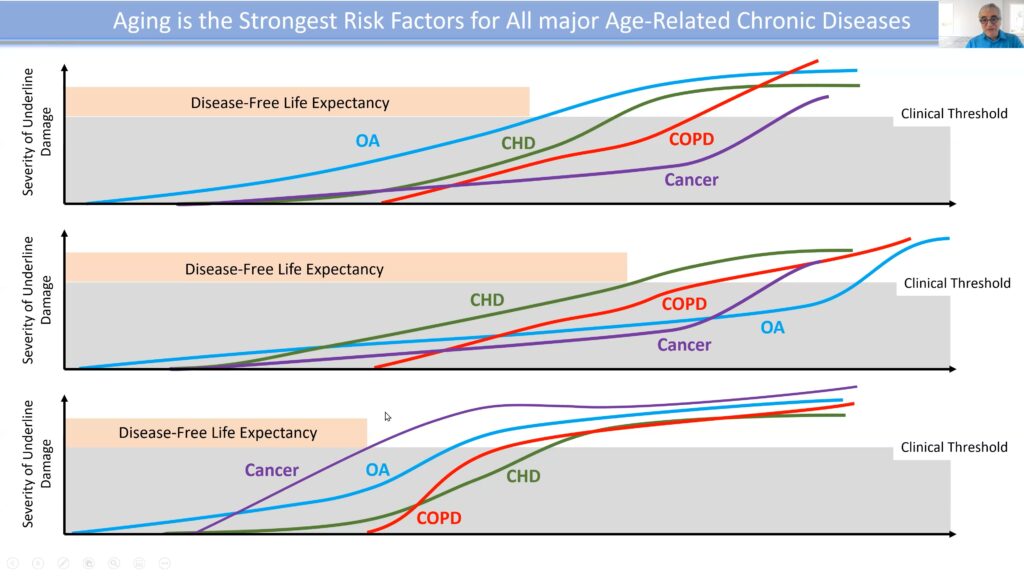
The rate of biological aging can be defined by the ratio of cellular damage accumulation to repair capacity. If the rate of damage accretion is fast, but the repair capacity is high, there won’t be an accumulation of damage, and aging will be slowed. However, when damage outpaces repair, aging accelerates.
Repair pathways require energy to operate effectively, and mitochondrial function declines dramatically with age. Ferrucci’s team discovered that this decline is associated with an upregulation of alternative splicing of mitochondrial proteins. Delving deeper into this mechanism, they applied gene set enrichment analysis to 5,325 RNAs with at least one splice variant significantly altered in response to changing mitochondrial function, as measured by AMPK and aging.
Among the top hits were GLUT4, VEGFA, IRS2, mTOR, PI3K, ULK1, ACC1, NRF2, and PGC1-α. Of note, the splice A variant of the topmost hit, VEGFA, appeared to be geronic, while the B variant appeared to be anti-geronic, with the ratio of these variants declining with age. Thus, alternative splicing is a method by which the body copes with energy decline due to mitochondrial dysfunction.
Further Readings
Promislow
Harrison BR, Wang L, Gajda E, et al.
BMC Genomics. 2020 May 4;21(1):341.
Jin K, Wilson KA, Beck JN, et al.
Laye MJ, Tran V, Jones DP, et al.
The effects of age and dietary restriction on the tissue-specific metabolome of Drosophila.
Aging Cell. 2015 Oct;14(5):797-808.
Hoffman JM, Ross C, Tran V, et al.
The metabolome as a biomarker of mortality risk in the common marmoset.
Am J Primatol. 2019 Feb;81(2):e22944.
Nelson PG, Promislow DEL, Masel J.
Biomarkers for Aging Identified in Cross-sectional Studies Tend to Be Non-causative.
J Gerontol A Biol Sci Med Sci. 2020 Feb 14;75(3):466-472.
Ferrucci
St Sauver JL, Boyd CM, Grossardt BR, et al.
BMJ Open. 2015 Feb 3;5(2):e006413.
Ubaida-Mohien C, Gonzalez-Freire M, Lyashkov A, et al.
Front Physiol. 2019;10:312.
Fabbri E, An Y, Zoli M, et al.
J Gerontol A Biol Sci Med Sci. 2015 Jan;70(1):63-70.
Choi S, Reiter DA, Shardell M, et al.
J Gerontol A Biol Sci Med Sci. 2016 Dec;71(12):1638-1645.
Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, et al.
Translational Research for Healthspan and Lifespan
Speakers
Pat Furlong, Panelist
Parent Project Muscular Distrophy
Roman J. Giger
University of Michigan School of Medicine
Senolytics: The Path to Translation
Physician-scientist James Kirkland studies the impact of cellular aging, specifically senescence, on age-related dysfunction and chronic diseases to develop methods for removing these cells and attenuating their deleterious effects. Senescent cells accumulate with aging and diseases, eliminating cells around them due to their senescence-associated secretory phenotype (SASP), which 30%-70% of senescent cells exhibit under most conditions.
Kirkland’s team applied a bioinformatics-based approach to analyze SASP proteomic databases, revealing that pro-survival networks are upregulated, with diverse senescent cells relying on different pathways. Several agents, termed senolytics, were identified that could target multiple nodes of these cascades.
“We’re moving away from the one drug, one target, one disease approach here,” said Kirkland, “to try and use agents that have multiple targets, or combinations of agents, to go after networks, and to go after senescent cells by doing this, and thereby improve…multiple conditions.”
Dasatinib (D), a SRC kinase inhibitor, preferentially killed senescent preadipocytes, which relied on survival pathways that signal through this kinase. Quercetin (Q) eliminated senescent human umbilical endothelial cells (HUVECs), which partly act through the Bcl-2 family and others that this cell type is susceptible to.
In an in vivo experiment, combining Dasatinib with Quercetin (D+Q) cleared transplanted luciferase-expressing senescent preadipocytes from mice, explicitly targeting those cells with a SASP. A single dose of senolytics also alleviated radiation-induced gait disturbance in mice, with the effects persisting long-term. Bi-weekly dosing reduced physical dysfunction in older mice, as measured by parameters of maximal speed, including treadmill and hanging endurance, grip strength, and daily activity, with D+Q significantly increasing performance across the board.
Many conditions have now been shown to be alleviated by various senolytics in a range of mouse models, with D+Q delaying death from all causes, and increasing healthspan and median lifespan.
Keynote: Mitochondrial-derived Peptides (MDPs) and the Regulation of Aging Processes
The discovery of mitochondrial peptides (MDPs), encoded from small genes less than 100 codons in length, established the birth and advancement of the microprotein subfield. Physician Pinchas Cohen works to understand mitochondrial biology and characterize MDPs, exploiting findings to target aging. MDPs are secreted from cells and circulate within the body.
“Overall, they serve as protective factors, or hormones if you will, that act in the brain, the heart, the liver, the muscle, and other organs,” Cohen stated.
Among these MDPs, Cohen’s lab identified humanin, encoded from the 16S region of mtDNA, and MOTS-c, encoded from the 12S region.
Humanin has a strong protective effect on neurons and against atherosclerosis, mitigates the side effects of chemotherapy while enhancing its benefit, and is related to longevity in model organisms and humans. Cohen’s lab employs mitochondrial-wide association studies (MiWAS) to link the dysfunction of MDPs to disease. MiWAS identified a single-nucleotide polymorphism (SNPs) in the humanin gene (rs2854128) associated with reduced levels and cognitive decline in humans and mice. Supplementing humanin in mice carrying this SNP improved their cognition.
MOTS-c is a novel exercise mimetic that has potential utility in numerous age-related diseases. Mice on a high fat diet receiving MOTS-c had dramatically lower weight compared to controls. MOTS-c treatment also improved exercise tolerance and performance in middle-aged and old mice, with older mice displaying the most dramatic improvement.
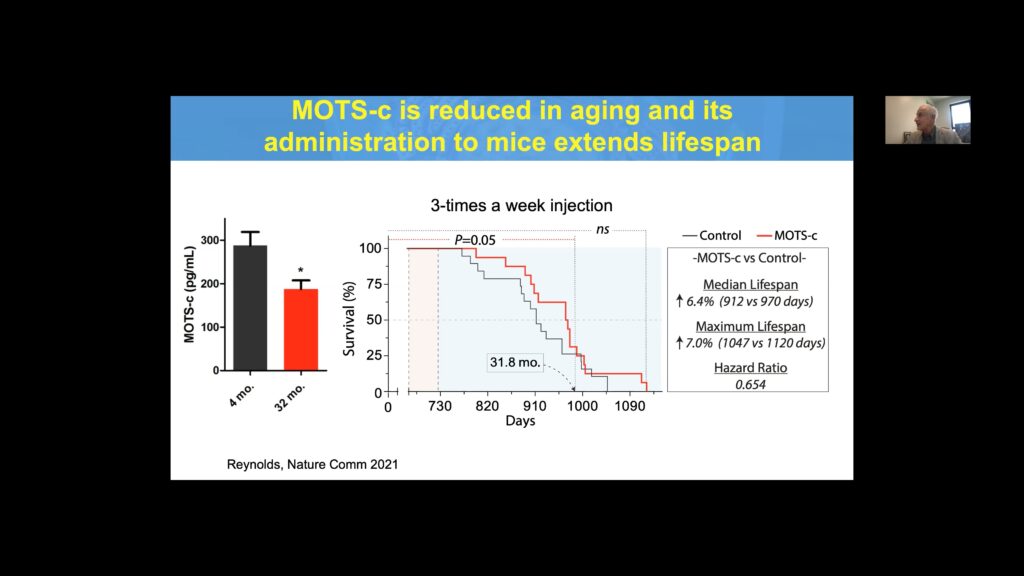
Cohen’s group also identified a link between a SNP in MOTS-c–K14Q–which nullifies MOTS-c activity and the risk of diabetes in males of the Asian population. Evaluating Japanese males from three cohorts revealed a 50% increase in the risk of diabetes for carriers, with almost double the risk seen exclusively in men who were sedentary. Like other MDPs, MOTS-c is reduced with age, and its administration to mice significantly extends lifespan.
“I think that everything we do in the aging field can be reduced to trying to simulate the beneficial effects of a healthy lifestyle, particularly diet…and exercise,” Cohen said. “We think that…mitochondria are the main source of action [here] by inducing the production of peptides such as MOTS-c, humanin, and others.”
Further Readings
Kirkland
Zhu Y, Tchkonia T, Pirtskhalava T, et al.
The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs.
Aging Cell. 2015 Aug;14(4):644-58.
Kirkland JL, Tchkonia T.
Senolytic drugs: from discovery to translation.
J Intern Med. 2020 Nov;288(5):518-536.
Ogrodnik M, Miwa S, Tchkonia T, et al.
Xu M, Pirtskhalava T, Farr JN, et al.
Senolytics improve physical function and increase lifespan in old age.
Justice JN, Nambiar AM, Tchkonia T, et al.
Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study.
EBioMedicine. 2019 Feb;40:554-563.
Cohen
Mehta HH, Xiao J, Ramirez R, et al.
Metabolomics. 2019 Jun 6;15(6):88.
Zempo H, Kim SJ, Fuku N, et al.
A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c.
Aging. 2021 Jan 19;13(2):1692-1717.
Yen K, Mehta HH, Kim SJ, et al.
The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan.
Aging. 2020 Jun 23;12(12):11185-11199.
Miller B, Kim SJ, Kumagai H, et al.
Exp Cell Res. 2020 Aug 15;393(2):112056.
Zempo H, Kim SJ, Fuku N, et al.
A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c.
Aging. 2021 Jan 19;13(2):1692-1717.